

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM223358 US9315520, Comparator 7::US9605007, Comparator 7::US9744173, Comparator 7
SMILES: [H][C@]12CSC(N)=N[C@]1(CO[C@@H](C)C2)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F
InChI Key: InChIKey=UOHXUSCZQJMWQO-PVUDRZGPSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM223358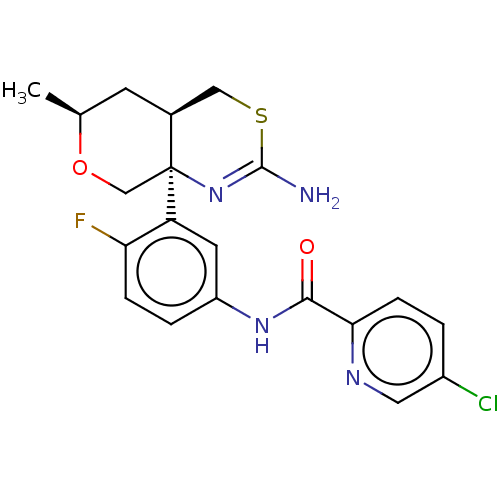 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description CYP2D6 inhibition was obtained by measuring inhibition of 5 uM Dextromethorphan in pooled HLM (HL-MIX-102). | US Patent US9315520 (2016) BindingDB Entry DOI: 10.7270/Q20000Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc. US Patent | Assay Description All testing was carried out in CHO cells transfected with the hERG gene purchased from Millipore (PrecisION hERG-CHO Recombinant Cell Line CYL3038). ... | US Patent US9315520 (2016) BindingDB Entry DOI: 10.7270/Q20000Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (BACE1) (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 37 |
Pfizer Inc. US Patent | Assay Description The substrate is Biotin-GLTNIKTEEISEISY^EVEFR-C[Oregon Green]KK-OH. The BACE1 enzyme is affinity purified material from conditioned media of CHO-K1 c... | US Patent US9315520 (2016) BindingDB Entry DOI: 10.7270/Q20000Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description CYP2D6 inhibition was obtained by measuring inhibition of 5 uM Dextromethorphan in pooled HLM (HL-MIX-102). | US Patent US9744173 (2017) BindingDB Entry DOI: 10.7270/Q2K076CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description CYP2D6 inhibition was obtained by measuring inhibition of 5 uM Dextromethorphan in pooled HLM (HL-MIX-102). | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description All testing was carried out in CHO cells transfected with the hERG gene purchased from Millipore (PrecisION hERG-CHO Recombinant Cell Line CYL3038). ... | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Beta-secretase (BACE) is one of the enzymes involved in the generation of the amyloid beta peptide found in the amyloid plaques of Alzheimer's Di... | US Patent US9744173 (2017) BindingDB Entry DOI: 10.7270/Q2K076CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223358 (US9315520, Comparator 7 | US9605007, Comparator 7 ...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The BACE1 and BACE2 binding assays measured beta-site amyloid precursor protein-cleaving enzyme (BACE) binding as a decrease in the counts of radioli... | US Patent US9605007 (2017) BindingDB Entry DOI: 10.7270/Q2JQ133S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||