

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22568 1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]ethyl}-2-methylguanidine::[3H]tiotidine::tiotidine
SMILES: [#6]-[#7]-[#6](-[#7]C#N)=[#7]-[#6]-[#6]-[#16]-[#6]-c1csc(\[#7]=[#6](\[#7])-[#7])n1
InChI Key: InChIKey=YDDXVAXDYKBWDX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H2 Receptor (Homo sapiens (Human)) | BDBM22568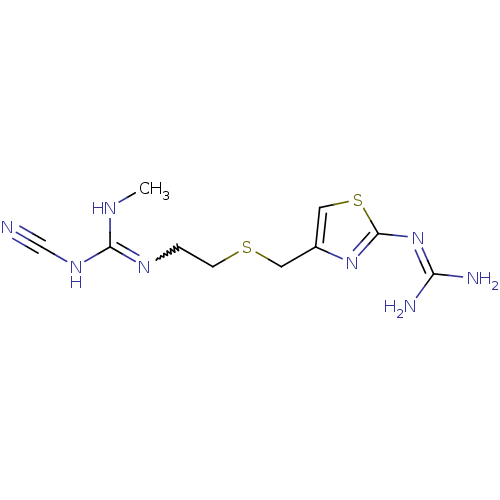 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Aminopotentidine from human recombinant histamine H2 receptor expressed in CHOK1 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22568 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22568 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Pharmaceutical Research Laboratories Curated by PDSP Ki Database | Nature 304: 65-7 (1983) Article DOI: 10.1038/304065a0 BindingDB Entry DOI: 10.7270/Q2930RN2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HRH3 (RAT) | BDBM22568 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by PDSP Ki Database | J Neurochem 55: 1612-6 (1990) Article DOI: 10.1111/j.1471-4159.1990.tb04946.x BindingDB Entry DOI: 10.7270/Q2X63KF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22568 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22568 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Brain Res 304: 1-7 (1984) Article DOI: 10.1016/0006-8993(84)90856-4 BindingDB Entry DOI: 10.7270/Q21N7ZMS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 Receptor (Homo sapiens (Human)) | BDBM22568 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Aminopotentidine from human recombinant histamine H2 receptor expressed in CHOK1 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||