

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22725 Butan-2,3-dione::butane-2,3-dione
SMILES: CC(=O)C(C)=O
InChI Key: InChIKey=QSJXEFYPDANLFS-UHFFFAOYSA-N
Data: 10 KI
PDB links: 1 PDB ID matches this monomer. 4 PDB IDs contain this monomer as substructures. 4 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor XIIa (Homo sapiens (Human)) | BDBM22725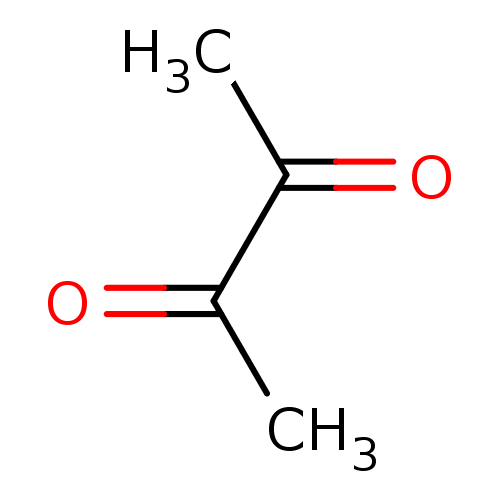 (Butan-2,3-dione | butane-2,3-dione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | >-5.45 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22725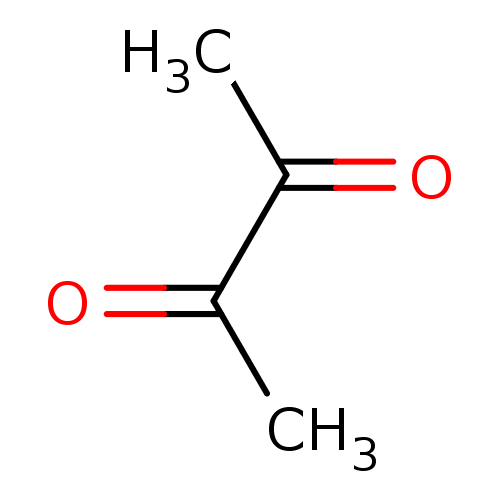 (Butan-2,3-dione | butane-2,3-dione) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22725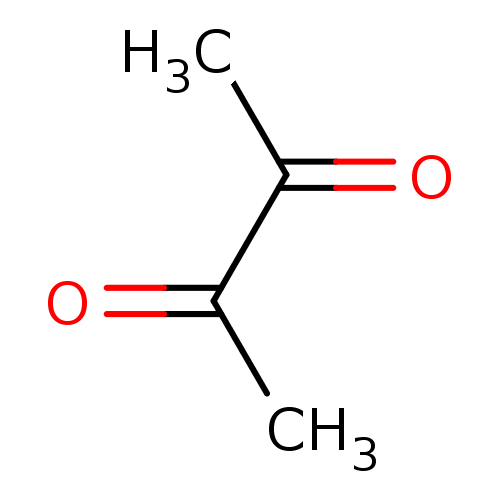 (Butan-2,3-dione | butane-2,3-dione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22725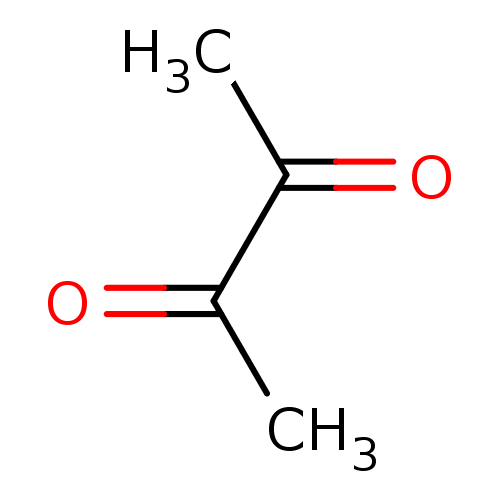 (Butan-2,3-dione | butane-2,3-dione) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22725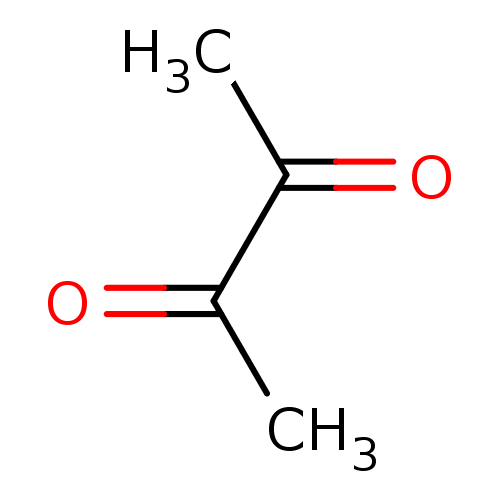 (Butan-2,3-dione | butane-2,3-dione) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 19: 4635-43 (2011) Article DOI: 10.1016/j.bmc.2011.06.012 BindingDB Entry DOI: 10.7270/Q2PR7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22725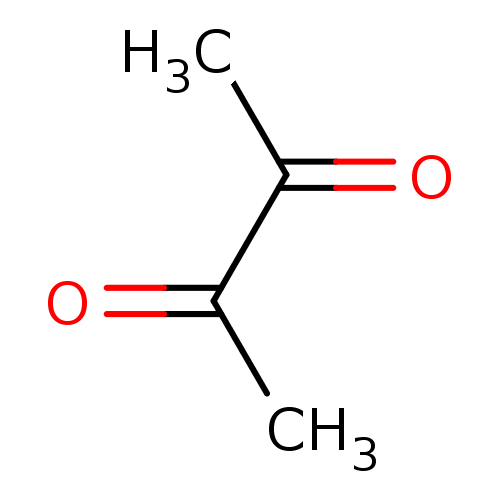 (Butan-2,3-dione | butane-2,3-dione) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human liver carboxylesterase1 using o-nitrophenyl acetate as substrate after 5 mins by spectrophotometry | Bioorg Med Chem 19: 4635-43 (2011) Article DOI: 10.1016/j.bmc.2011.06.012 BindingDB Entry DOI: 10.7270/Q2PR7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM22725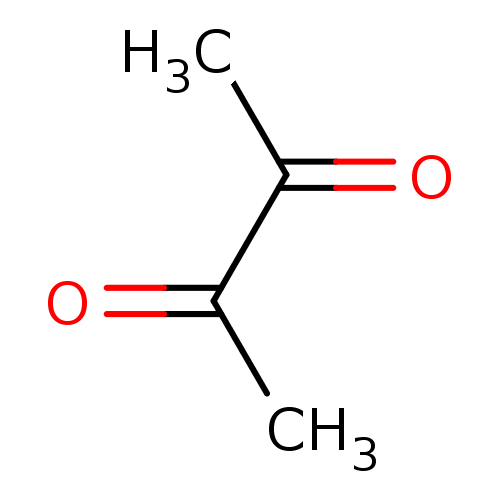 (Butan-2,3-dione | butane-2,3-dione) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human butyrylcholinesterase using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 19: 4635-43 (2011) Article DOI: 10.1016/j.bmc.2011.06.012 BindingDB Entry DOI: 10.7270/Q2PR7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase 2 (Homo sapiens (Human)) | BDBM22725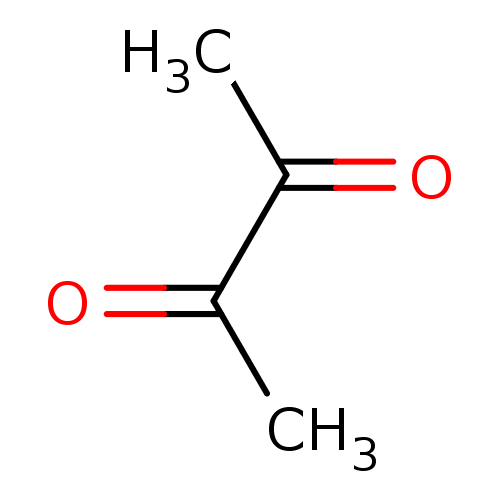 (Butan-2,3-dione | butane-2,3-dione) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human intestinal carboxylesterase using o-nitrophenyl acetate as substrate after 5 mins by spectrophotometry | Bioorg Med Chem 19: 4635-43 (2011) Article DOI: 10.1016/j.bmc.2011.06.012 BindingDB Entry DOI: 10.7270/Q2PR7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22725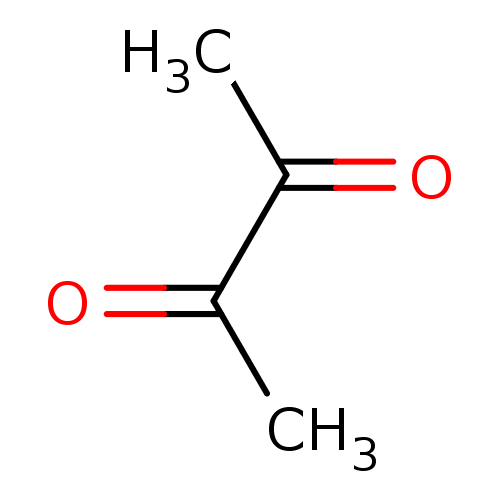 (Butan-2,3-dione | butane-2,3-dione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of rabbit liver carboxylesterase using o-nitrophenyl acetate as substrate after for 5 mins by spectrophotometry | Bioorg Med Chem 19: 4635-43 (2011) Article DOI: 10.1016/j.bmc.2011.06.012 BindingDB Entry DOI: 10.7270/Q2PR7WB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM22725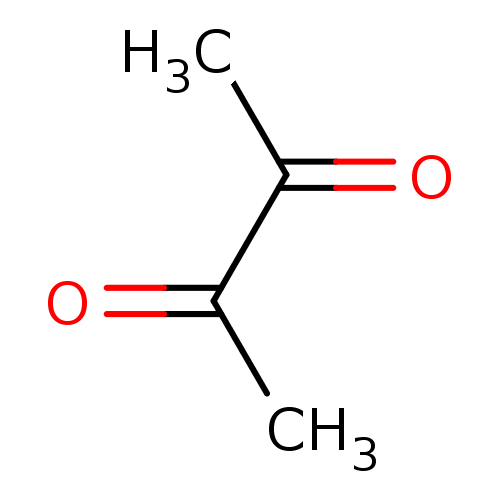 (Butan-2,3-dione | butane-2,3-dione) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||