

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM227458 US9333217, 75
SMILES: N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O
InChI Key: InChIKey=WWKSYRSOVZNFOY-XGJLOEHPSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458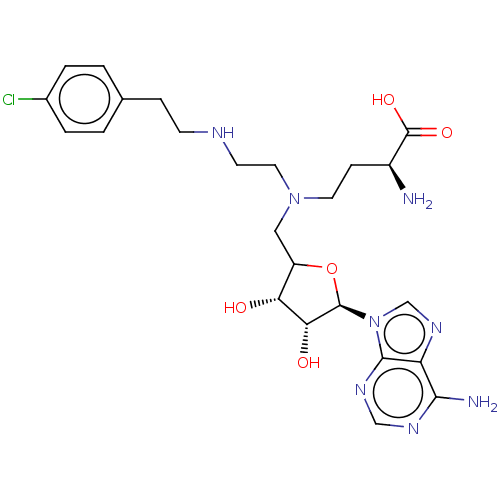 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM227458 (US9333217, 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc. US Patent | Assay Description Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... | US Patent US9333217 (2016) BindingDB Entry DOI: 10.7270/Q2XP73TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||