

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22751 1,2-bis(3-nitrophenyl)ethane-1,2-dione::Benzil-based compound, 29
SMILES: [O-][N+](=O)c1cccc(c1)C(=O)C(=O)c1cccc(c1)[N+]([O-])=O
InChI Key: InChIKey=SGKVXDKTNJHBCA-UHFFFAOYSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22751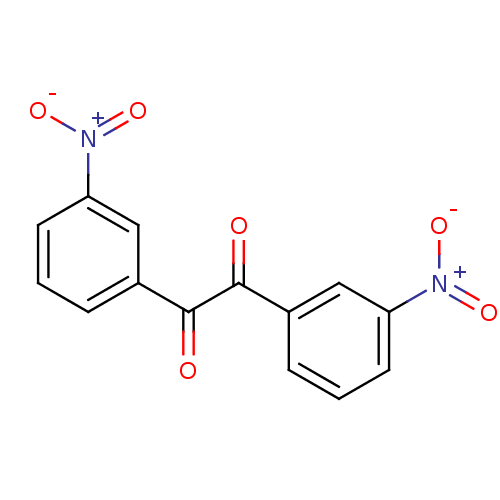 (1,2-bis(3-nitrophenyl)ethane-1,2-dione | Benzil-ba...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor XIIa (Homo sapiens (Human)) | BDBM22751 (1,2-bis(3-nitrophenyl)ethane-1,2-dione | Benzil-ba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 397 | -8.73 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22751 (1,2-bis(3-nitrophenyl)ethane-1,2-dione | Benzil-ba...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22751 (1,2-bis(3-nitrophenyl)ethane-1,2-dione | Benzil-ba...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of human carboxylesterase 1 | Bioorg Med Chem 17: 149-64 (2008) Article DOI: 10.1016/j.bmc.2008.11.008 BindingDB Entry DOI: 10.7270/Q2JH3NFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22751 (1,2-bis(3-nitrophenyl)ethane-1,2-dione | Benzil-ba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM22751 (1,2-bis(3-nitrophenyl)ethane-1,2-dione | Benzil-ba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||