

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22774 1,4-Benzoquinone::Benzil-related compound, 53::CHEMBL8320::benzoquinone::cid_4650::cyclohexa-2,5-diene-1,4-dione
SMILES: O=C1C=CC(=O)C=C1
InChI Key: InChIKey=AZQWKYJCGOJGHM-UHFFFAOYSA-N
Data: 7 KI 10 IC50 1 Kd 1 Other
PDB links: 6 PDB IDs match this monomer. 107 PDB IDs contain this monomer as substructures. 107 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM22774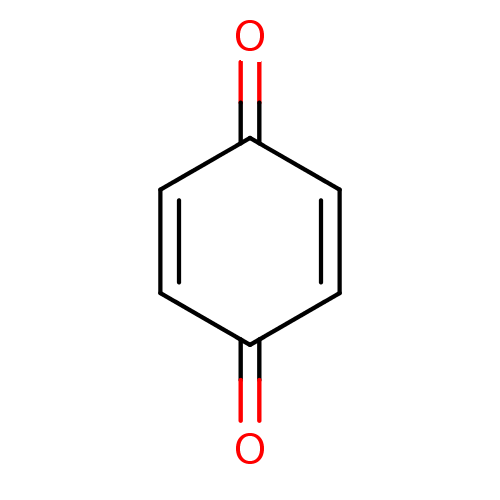 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 9.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry, School of Pharmacy, Centre of Excellence for Pharmaceutical Sciences, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa. Curated by ChEMBL | Assay Description Competitive inhibition of human brain mitochondria MAO-A using kynuramine as substrate by Lineweaver-Burk analysis | Eur J Med Chem 135: 196-203 (2017) Article DOI: 10.1016/j.ejmech.2017.04.055 BindingDB Entry DOI: 10.7270/Q2MW2KM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry, School of Pharmacy, Centre of Excellence for Pharmaceutical Sciences, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa. Curated by ChEMBL | Assay Description Non-competitive inhibition of human brain mitochondria MAO-B using kynuramine as substrate by Lineweaver-Burk analysis | Eur J Med Chem 135: 196-203 (2017) Article DOI: 10.1016/j.ejmech.2017.04.055 BindingDB Entry DOI: 10.7270/Q2MW2KM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase 1 (Oryctolagus cuniculus (rabbit)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor XIIa (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... | J Med Chem 48: 2906-15 (2005) Article DOI: 10.1021/jm049011j BindingDB Entry DOI: 10.7270/Q2ZP44DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MDM2-MDMX (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of biotin-labelled p53 binding to MDM2 (25 to 109 residues) (unknown origin) by surface plasmon resonance method | Bioorg Med Chem Lett 27: 2571-2574 (2017) Article DOI: 10.1016/j.bmcl.2017.03.082 BindingDB Entry DOI: 10.7270/Q2571F44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry, School of Pharmacy, Centre of Excellence for Pharmaceutical Sciences, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cell microsomes using kynuramine as substrate after 20 mins by flu... | Eur J Med Chem 135: 196-203 (2017) Article DOI: 10.1016/j.ejmech.2017.04.055 BindingDB Entry DOI: 10.7270/Q2MW2KM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry, School of Pharmacy, Centre of Excellence for Pharmaceutical Sciences, North-West University, Private Bag X6001, Potchefstroom 2520, South Africa. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI insect cell microsomes using kynuramine as substrate after 20 mins by flu... | Eur J Med Chem 135: 196-203 (2017) Article DOI: 10.1016/j.ejmech.2017.04.055 BindingDB Entry DOI: 10.7270/Q2MW2KM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Schistosoma mansoni) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay | Eur J Med Chem 167: 357-366 (2019) Article DOI: 10.1016/j.ejmech.2019.02.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MKP-3 (Rattus norvegicus) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged rat recombinant MKP3 catalytic domain expressed in Escherichia coli BL21(DE3) | Bioorg Med Chem 17: 2276-81 (2009) Article DOI: 10.1016/j.bmc.2008.10.090 BindingDB Entry DOI: 10.7270/Q21J99MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 1 (Mus musculus) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged mouse MKP1 catalytic domain expressed in human Hela cells | Bioorg Med Chem 17: 2276-81 (2009) Article DOI: 10.1016/j.bmc.2008.10.090 BindingDB Entry DOI: 10.7270/Q21J99MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity phosphatase Cdc25B (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of histidine-tagged human recombinant Cdc25B catalytic domain expressed in Escherichia coli | Bioorg Med Chem 17: 2276-81 (2009) Article DOI: 10.1016/j.bmc.2008.10.090 BindingDB Entry DOI: 10.7270/Q21J99MN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhóne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against IL-1 beta converting enzyme was evaluated. | Bioorg Med Chem Lett 8: 1883-6 (1999) BindingDB Entry DOI: 10.7270/Q2X929FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbon monoxide dehydrogenase (CODH) (Oligotropha carboxidovorans) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a | n/a |
University of California, Riverside | Assay Description Inhibition of CODH by Diphenyliodomium chloride. Inactivation of the FAD cofactor of CODH was accomplished by covalent modification of the flavin wi... | Biochemistry 50: 1910-6 (2011) Article DOI: 10.1021/bi1017182 BindingDB Entry DOI: 10.7270/Q27M06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA dC->dU-editing enzyme APOBEC-3G (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | PCBioAssay | n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2HQ3XD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of human DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay | Eur J Med Chem 167: 357-366 (2019) Article DOI: 10.1016/j.ejmech.2019.02.018 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM22774 (1,4-Benzoquinone | Benzil-related compound, 53 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||