 Found 14 hits for monomerid = 22877
Found 14 hits for monomerid = 22877 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22877
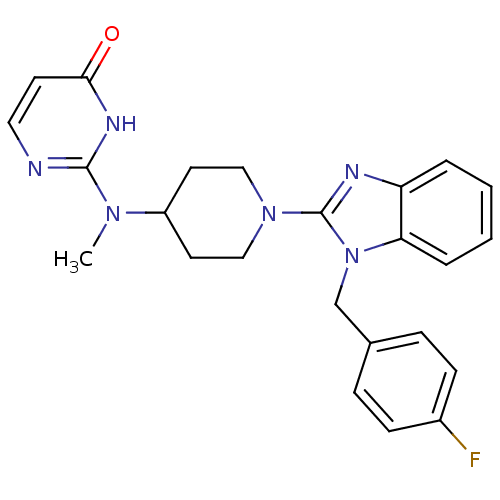
(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetolide from human ERG channel expressed in HEK293 cells at 37 degC by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | >-7.09 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human Potassium channel HERG expressed in mammalian cells |
Bioorg Med Chem Lett 13: 2773-5 (2003)
BindingDB Entry DOI: 10.7270/Q2QZ2BGZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
K+ channel blocking activity in human embryonic kidney cells expressing HERG Kv11.1 |
J Med Chem 45: 3844-53 (2002)
BindingDB Entry DOI: 10.7270/Q2NC61X3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of partially open human voltage-gated potassium channel subunit Kv11.1 (ERG K+ channel) |
Bioorg Med Chem Lett 15: 1737-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.008
BindingDB Entry DOI: 10.7270/Q2FT8NCR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human ERG in MCF7 cells |
Eur J Med Chem 44: 1926-32 (2009)
Article DOI: 10.1016/j.ejmech.2008.11.009
BindingDB Entry DOI: 10.7270/Q2TM7CCD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Reverse proteomics research institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against potassium channel HERG |
Bioorg Med Chem Lett 15: 2886-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.080
BindingDB Entry DOI: 10.7270/Q29S1S7C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by whole cell patch clamp technique |
Bioorg Med Chem 16: 6252-60 (2008)
Article DOI: 10.1016/j.bmc.2008.04.028
BindingDB Entry DOI: 10.7270/Q25D8T25 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by patch clamp assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22877

(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data