

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22884 2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine::2-[3-(1H-imidazol-5-yl)propyl]-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine::IMPROMIDINE::Impromidine
SMILES: Cc1nc[nH]c1CSCCN=C(N)NCCCc1cnc[nH]1
InChI Key: InChIKey=MURRAGMMNAYLNA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22884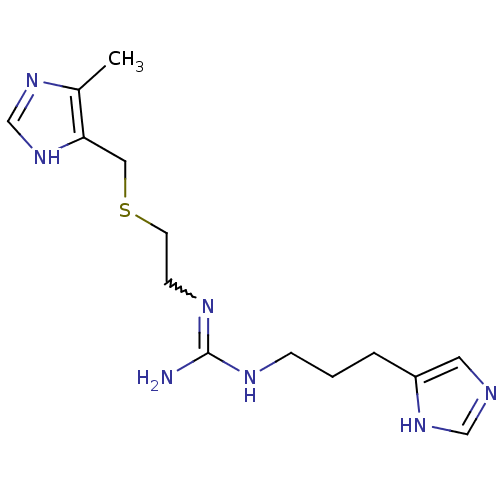 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H4 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research & Development Ltd. Curated by PDSP Ki Database | Eur J Pharmacol 311: 305-10 (1996) Article DOI: 10.1016/0014-2999(96)00428-1 BindingDB Entry DOI: 10.7270/Q2ZG6QSD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research & Development Ltd. Curated by PDSP Ki Database | Eur J Pharmacol 311: 305-10 (1996) Article DOI: 10.1016/0014-2999(96)00428-1 BindingDB Entry DOI: 10.7270/Q2ZG6QSD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Binding affinity towards Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Curated by PDSP Ki Database | J Neurochem 55: 1612-6 (1990) Article DOI: 10.1111/j.1471-4159.1990.tb04946.x BindingDB Entry DOI: 10.7270/Q2X63KF2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Pharmaceutical Research Laboratories Curated by PDSP Ki Database | Nature 304: 65-7 (1983) Article DOI: 10.1038/304065a0 BindingDB Entry DOI: 10.7270/Q2930RN2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonistic activity tested against Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by PDSP Ki Database | Mol Pharmacol 59: 420-6 (2001) Article DOI: 10.1124/mol.59.3.420 BindingDB Entry DOI: 10.7270/Q26Q1VSR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to the human histamine H3 receptor | J Med Chem 46: 3957-60 (2003) Article DOI: 10.1021/jm0341047 BindingDB Entry DOI: 10.7270/Q2QJ7J1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as inhibition of histamine-induced positive chronotropic activity | J Med Chem 51: 7193-204 (2009) Article DOI: 10.1021/jm800841w BindingDB Entry DOI: 10.7270/Q2RN38T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22884 (2-[3-(1H-imidazol-5-yl)propyl]-1-(2-{[(5-methyl-1H...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at histamine H2 receptor in guinea pig spontaneously beating right atrium assessed as positive chronotropic activity | J Med Chem 51: 7193-204 (2009) Article DOI: 10.1021/jm800841w BindingDB Entry DOI: 10.7270/Q2RN38T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||