

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22916 5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole::CHEMBL19010::Iodoproxyfan
SMILES: Ic1ccc(COCCCc2cnc[nH]2)cc1
InChI Key: InChIKey=DCQNAFOCXVCDLB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRH3 (RAT) | BDBM22916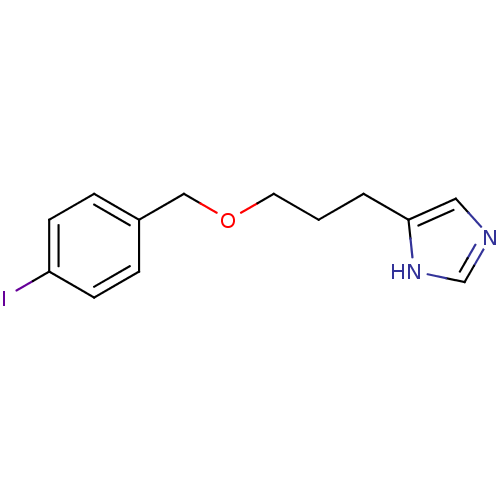 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HRH3 (RAT) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Binding affinity towards Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonistic activity tested against Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HRH3 (RAT) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U. 109 Curated by PDSP Ki Database | J Pharmacol Exp Ther 271: 452-9 (1994) BindingDB Entry DOI: 10.7270/Q23X8556 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description In vitro antagonistic activity tested against Histamine H3 receptor on synaptosomes from rat cerebral cortex | J Med Chem 39: 1220-6 (1996) Article DOI: 10.1021/jm9504767 BindingDB Entry DOI: 10.7270/Q28K7855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting | J Med Chem 54: 8136-47 (2011) Article DOI: 10.1021/jm201042n BindingDB Entry DOI: 10.7270/Q2MW2JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam | Assay Description Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... | J Pharmacol Exp Ther 314: 1310-21 (2005) Article DOI: 10.1124/jpet.105.087965 BindingDB Entry DOI: 10.7270/Q2KD1W6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan binding to human histamine H3 receptor of CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Inhibition of [125I]-iodoproxyfan from rat histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 11: 951-4 (2001) BindingDB Entry DOI: 10.7270/Q28P5ZRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HRH3 (GUINEA PIG) | BDBM22916 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex | J Med Chem 44: 1666-74 (2001) BindingDB Entry DOI: 10.7270/Q2WH2QP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||