

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23027 1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-2-yl)heptan-1-one::alpha-ketooxazole, 5a
SMILES: O=C(CCCCCCc1cccs1)c1ncc(o1)-c1ccccn1
InChI Key: InChIKey=VYGSYWKKZIMLJG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid Amide Hydrolase (Homo sapiens (Human)) | BDBM23027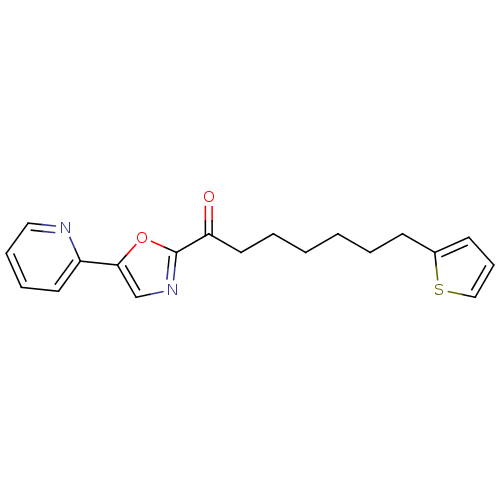 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | -11.3 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Triacylglycerol Hydrolase (Homo sapiens (Human)) | BDBM23027 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||