

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23073 3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]propan-1-one::CHEMBL226938::alpha-ketooxazole, 11e
SMILES: O=C(CCc1ccc(COc2ccccc2)cc1)c1ncc(o1)-c1ccccn1
InChI Key: InChIKey=ONDWZFFDWRKXKE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid Amide Hydrolase (Homo sapiens (Human)) | BDBM23073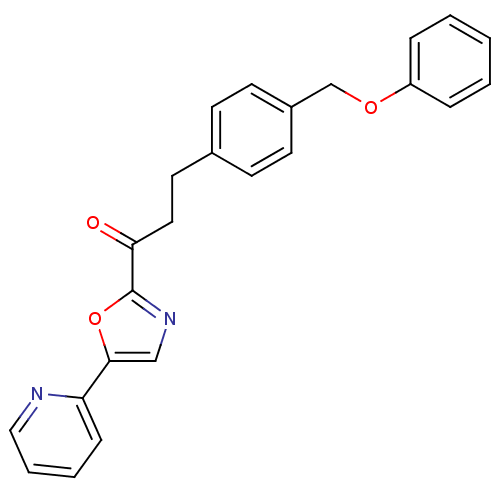 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -12.3 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of rat recombinant FAAH expressed in Escherichia coli | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of FAAH | J Med Chem 51: 7327-43 (2009) Article DOI: 10.1021/jm800311k BindingDB Entry DOI: 10.7270/Q2J67HT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of rat recombinant FAAH expressed in Escherichia coli | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH expressed COS7 cells | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -11.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA: cholesterol acyltransferase (ACAT) (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of rat recombinant FAAH expressed in Escherichia coli | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral cholesterol ester hydrolase 1 (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of KIAA1363 | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Triacylglycerol Hydrolase (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||