

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM231662 DMeCF3 (13)::US10668094, Compound DCF3
SMILES: C[C@@H]1O[C@H](C[C@H](O)[C@@H]1O[C@H]1C[C@H](O)[C@H](OC2CCN(CC(F)(F)F)CC(C)O2)[C@H](C)O1)O[C@H]1CC[C@@]2(C)C(CCC3C2C[C@H](O)[C@]2(C)[C@H](CC[C@]32O)C2=CC(=O)OC2)C1
InChI Key: InChIKey=UNYFXQCNOFHMAF-NVAUYEMTSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na,K-ATPase alpha2beta3 (Homo sapiens (Human)) | BDBM231662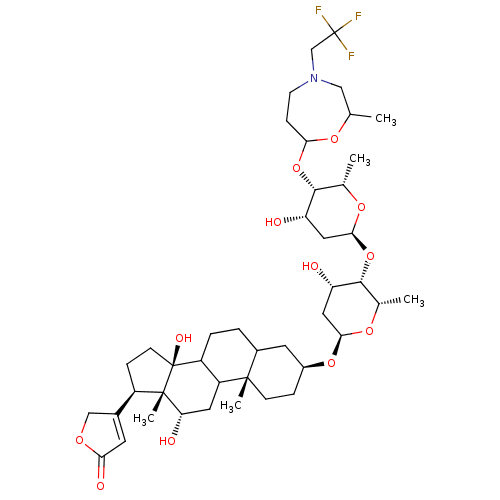 (DMeCF3 (13) | US10668094, Compound DCF3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Na,K-ATPase alpha2beta2 (Homo sapiens (Human)) | BDBM231662 (DMeCF3 (13) | US10668094, Compound DCF3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Na,K-ATPase alpha2beta1 (Homo sapiens (Human)) | BDBM231662 (DMeCF3 (13) | US10668094, Compound DCF3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Na,K-ATPase alpha2beta1 (Homo sapiens (Human)) | BDBM231662 (DMeCF3 (13) | US10668094, Compound DCF3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weizmann Institute of Science | Assay Description Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by CGs was done exactly as d... | J Biol Chem 289: 21153-62 (2014) Article DOI: 10.1074/jbc.M114.557629 BindingDB Entry DOI: 10.7270/Q2G44P6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Na,K-ATPase alpha1beta1 (Homo sapiens (Human)) | BDBM231662 (DMeCF3 (13) | US10668094, Compound DCF3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Na,K-ATPase alpha1beta1 (Homo sapiens (Human)) | BDBM231662 (DMeCF3 (13) | US10668094, Compound DCF3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Weizmann Institute of Science | Assay Description Inhibition of Na,K-ATPase activity of the detergent-soluble α1β1, α2β1, and α3β1 complexes by CGs was done exactly as d... | J Biol Chem 289: 21153-62 (2014) Article DOI: 10.1074/jbc.M114.557629 BindingDB Entry DOI: 10.7270/Q2G44P6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||