

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM234401 Oleandomycin
SMILES: CO[C@H]1C[C@H](O[C@H]2[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@@H](C)C[C@@]3(CO3)C(=O)[C@H](C)[C@@H](O)[C@@H](C)[C@@H](C)OC(=O)[C@@H]2C)O[C@@H](C)[C@@H]1O
InChI Key: InChIKey=RZPAKFUAFGMUPI-QESOVKLGSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uridine-5'-diphosphoglucuronosyltransferase 1A1 (Homo sapiens (Human)) | BDBM234401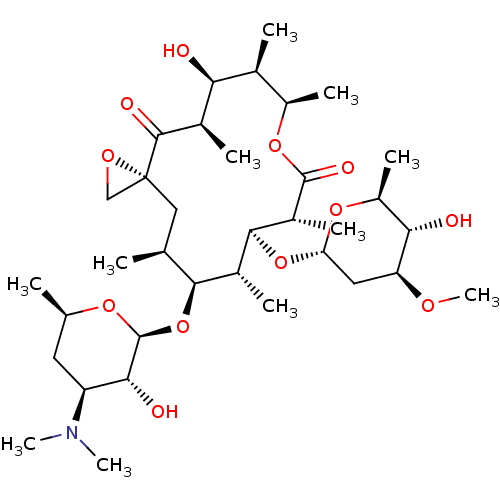 (Oleandomycin) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine-5'-diphosphoglucuronosyltransferase 1A4 (Homo sapiens (Human)) | BDBM234401 (Oleandomycin) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine-5'-diphosphoglucuronosyltransferase 2B10 (Homo sapiens (Human)) | BDBM234401 (Oleandomycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine-5'-diphosphoglucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM234401 (Oleandomycin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine-5'-diphosphoglucuronosyltransferase 1A6 (Homo sapiens (Human)) | BDBM234401 (Oleandomycin) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description Refer to Fisher et al., Drug Metab. Dispos., 28:560-566. | J Enzyme Inhib Med Chem 26: 386-93 (2011) Article DOI: 10.3109/14756366.2010.518965 BindingDB Entry DOI: 10.7270/Q2HQ3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||