

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23556 3-Carboxamide-coumarin deriv., 32::N-(3-chlorophenyl)-6-methyl-2-oxo-2H-chromene-3-carboxamide
SMILES: Cc1ccc2oc(=O)c(cc2c1)C(=O)Nc1cccc(Cl)c1
InChI Key: InChIKey=OOLBPPCJUQNAAW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM23556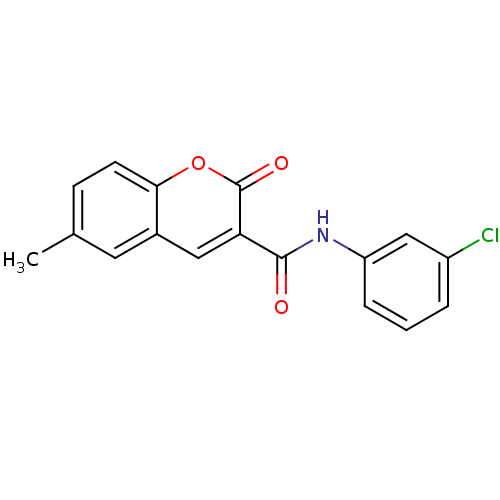 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto , 4169-007 Porto, Portugal. Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant microsomal MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate as... | J Med Chem 60: 7206-7212 (2017) Article DOI: 10.1021/acs.jmedchem.7b00918 BindingDB Entry DOI: 10.7270/Q23B62KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23556 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur | Assay Description After substrate hydrolysis reaction, the absorbance at 405 nm was measured in a microplate reader. Percentages of inhibition at each concentration w... | J Med Chem 51: 3077-80 (2008) Article DOI: 10.1021/jm8002697 BindingDB Entry DOI: 10.7270/Q2610XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM23556 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur | Assay Description After substrate hydrolysis reaction, the absorbance at 405 nm was measured in a microplate reader. Percentages of inhibition at each concentration we... | J Med Chem 51: 3077-80 (2008) Article DOI: 10.1021/jm8002697 BindingDB Entry DOI: 10.7270/Q2610XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM23556 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto , 4169-007 Porto, Portugal. Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decre... | J Med Chem 60: 7206-7212 (2017) Article DOI: 10.1021/acs.jmedchem.7b00918 BindingDB Entry DOI: 10.7270/Q23B62KQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM23556 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur | Assay Description After substrate hydrolysis reaction, the absorbance at 405 nm was measured in a microplate reader. Percentages of inhibition at each concentration we... | J Med Chem 51: 3077-80 (2008) Article DOI: 10.1021/jm8002697 BindingDB Entry DOI: 10.7270/Q2610XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor XIIa (Homo sapiens (Human)) | BDBM23556 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | 7.9 | 22 |
University of Namur | Assay Description After substrate hydrolysis reaction, the absorbance at 405 nm was measured in a microplate reader. Percentages of inhibition at each concentration we... | J Med Chem 51: 3077-80 (2008) Article DOI: 10.1021/jm8002697 BindingDB Entry DOI: 10.7270/Q2610XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM23556 (3-Carboxamide-coumarin deriv., 32 | N-(3-chlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur | Assay Description After substrate hydrolysis reaction, the absorbance at 405 nm was measured in a microplate reader. Percentages of inhibition at each concentration we... | J Med Chem 51: 3077-80 (2008) Article DOI: 10.1021/jm8002697 BindingDB Entry DOI: 10.7270/Q2610XNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||