

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM236591 US9365589, 1r
SMILES: CC1(C)C(=N)N[C@]2(COC[C@H]2S1(=O)=O)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F
InChI Key: InChIKey=ZRQWZDZYZAYTLY-DYESRHJHSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM236591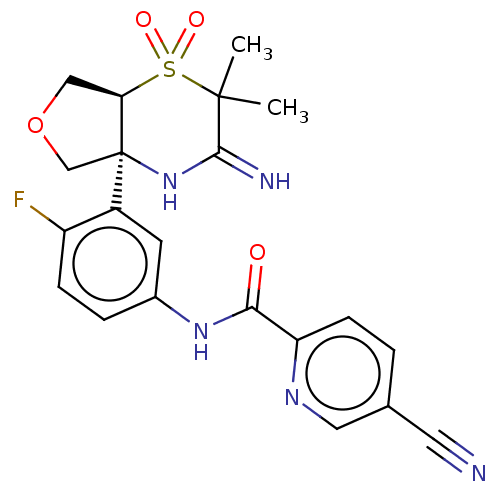 (US9365589, 1r) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -12.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description This assay monitors the increase of 620 nm fluorescence that resulted from BACE1 cleavage of an APPswedish APPswe mutant peptide FRET substrate (QSY7... | US Patent US9365589 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM236591 (US9365589, 1r) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of human BACE2 preincubated for 30 mins followed by QSY7- EISEVNLDAEFC-Eu-amide substrate addition and measured after 1.5 hrs by HTRF assa... | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM236591 (US9365589, 1r) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of human BACE1 catalytic domain preincubated for 30 mins followed by QSY7-EISEVNLDAEFC-Europium-amide substrate addition and measured afte... | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM236591 (US9365589, 1r) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor IC50s at purified human autoBACE-2 are determined in a time-resolved endpoint proteolysis assay that measures hydrolysis of the QSY7-EISEVN... | US Patent US9365589 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||