

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM238345 US9388165, 1
SMILES: Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cccc2c(N)nccc12
InChI Key: InChIKey=JETCTCUSGZDEGL-UHFFFAOYSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-RAF (Y340D Y341D) (Homo sapiens (Human)) | BDBM238345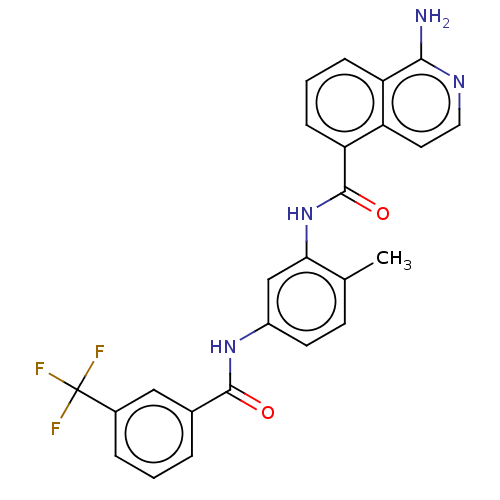 (US9388165, 1) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description The compounds prepared in Examples were tested for inhibitory activities against three subtypes of RAF, i.e. RAF1 Y340D Y341D, BRAF normal type and B... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-RAF V600E (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM238345 (US9388165, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO., LTD. US Patent | Assay Description In a similar manner to Experimental Example 1, the % inhibitory activity (concentration of the compound 1.0 μM) and the IC50 values of the inven... | US Patent US9388165 (2016) BindingDB Entry DOI: 10.7270/Q2F76BF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||