

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23840 2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindole-1,3-dione::CHEMBL246144::PP2P
SMILES: O=C1N(C(=O)c2ccccc12)c1ccccc1CCc1ccccc1
InChI Key: InChIKey=XGSWAPNRKGOSKD-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alpha-Glucosidase (alpha-Glu) (Saccharomyces cerevisiae) | BDBM23840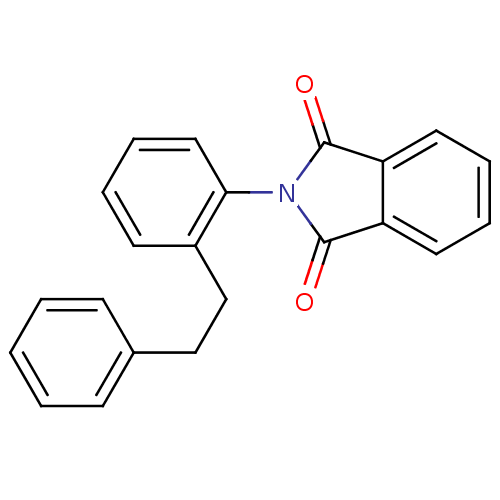 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LXRbeta expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activation by l... | Bioorg Med Chem Lett 17: 3957-61 (2007) Article DOI: 10.1016/j.bmcl.2007.04.090 BindingDB Entry DOI: 10.7270/Q2WS8SZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human LXRalpha expressed in HEK293 cells assessed as inhibition of beta-galactosidase activity by luciferase reporter gene ass... | Bioorg Med Chem 17: 5001-14 (2009) Article DOI: 10.1016/j.bmc.2009.05.066 BindingDB Entry DOI: 10.7270/Q2B56JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LXRalpha expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activation by ... | Bioorg Med Chem Lett 17: 3957-61 (2007) Article DOI: 10.1016/j.bmcl.2007.04.090 BindingDB Entry DOI: 10.7270/Q2WS8SZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human LXR-beta transfected in HEK293 cells after 16 hrs by luciferase reporter gene assay in presence of 0.1 uM N-(4-(1,1,1,3,... | ACS Med Chem Lett 6: 902-7 (2015) BindingDB Entry DOI: 10.7270/Q2VM4F2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human LXR-alpha transfected in HEK293 cells after 16 hrs by luciferase reporter gene assay in presence of 0.3 uM N-(4-(1,1,1,3... | ACS Med Chem Lett 6: 902-7 (2015) BindingDB Entry DOI: 10.7270/Q2VM4F2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human LXRbeta expressed in HEK293 cells assessed as inhibition of beta-galactosidase activity by luciferase reporter gene assa... | Bioorg Med Chem 17: 5001-14 (2009) Article DOI: 10.1016/j.bmc.2009.05.066 BindingDB Entry DOI: 10.7270/Q2B56JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||