

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc2CCc3ccc(cc3)-c3ccccc3CCc3ccc(Oc1c2)cc3
InChI Key: InChIKey=KPPHKUBDCAKMJR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM23847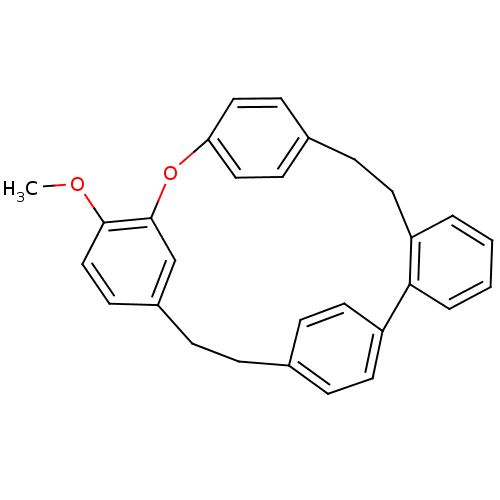 (16-methoxy-14-oxapentacyclo[20.2.2.2^{10,13}.1^{15...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23847 (16-methoxy-14-oxapentacyclo[20.2.2.2^{10,13}.1^{15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23847 (16-methoxy-14-oxapentacyclo[20.2.2.2^{10,13}.1^{15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||