

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM239160 α-CA inhibitor, 8
SMILES: CC(C)(O)[C@@H]1CC[C@@](C)(O1)[C@H]1[C@@H](O)C[C@@]2(C)C3C[C@H](O[C@@H]4OC(CO)[C@@H](O)C(O)[C@@H]4O)C4[C@]5(C[C@@]35CC[C@]12C)CC[C@H](O[C@@H]1OC[C@@H](O)C(O)[C@@H]1O)C4(C)C
InChI Key: InChIKey=QMNWISYXSJWHRY-CDDJQPGQSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM239160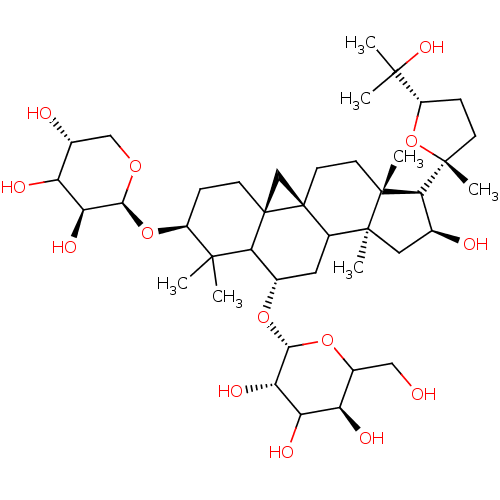 (α-CA inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM239160 (α-CA inhibitor, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (CA III) (Bos taurus (Cattle)) | BDBM239160 (α-CA inhibitor, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239160 (α-CA inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (CA-VI) (Homo sapiens (Human)) | BDBM239160 (α-CA inhibitor, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||