

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23928 1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]benzene::CHEMBL296411::trans-Stilbene Derivative, 4a
SMILES: COc1ccc(\C=C\c2cc(OC)cc(OC)c2)cc1
InChI Key: InChIKey=GDHNBPHYVRHYCC-SNAWJCMRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aryl Hydrocarbon Receptor (AhR) (Oryctolagus cuniculus (rabbit)) | BDBM23928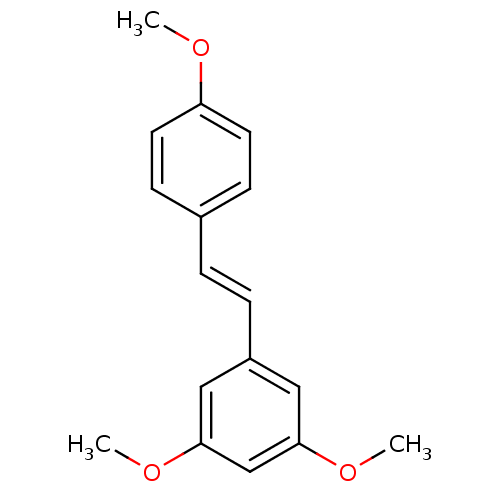 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 4 |
Institut Claudius Regaud | Assay Description Cytosols from rabbit liver were incubated with [3H]-TCDD and 12 concentrations of unlabeled test ligands. IC50 values were determined using the itera... | J Med Chem 48: 287-91 (2005) Article DOI: 10.1021/jm0498194 BindingDB Entry DOI: 10.7270/Q2WM1BQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description Cytosols from MCF-7 cells expressed ER-alpha isoform were incubated with [3H]-estradiol and eight concentrations of unlabeled test ligands. IC50 val... | J Med Chem 48: 287-91 (2005) Article DOI: 10.1021/jm0498194 BindingDB Entry DOI: 10.7270/Q2WM1BQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.30E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to aryl hydrocarbon receptor (unknown origin) | Citation and Details Article DOI: 10.1007/s00044-007-9055-2 BindingDB Entry DOI: 10.7270/Q2M32ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes epressing human cytochrome P450 1A1 | J Med Chem 45: 160-4 (2001) BindingDB Entry DOI: 10.7270/Q2668CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced increase of intracellular calcium level | Bioorg Med Chem Lett 26: 899-902 (2016) BindingDB Entry DOI: 10.7270/Q2765H6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Agonist activity at rat TRPA1 expressed in HEK293 cells assessed as induction of intracellular calcium level | Bioorg Med Chem Lett 26: 899-902 (2016) BindingDB Entry DOI: 10.7270/Q2765H6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "L. Vanvitelli" Curated by ChEMBL | Assay Description Inhibition of 5-LOX in human PMNL cells assessed as A23187-stimulated LTB4 production preincubated for 15 mins followed by A23187 addition and measur... | Eur J Med Chem 180: 637-647 (2019) Article DOI: 10.1016/j.ejmech.2019.07.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate assessed as PGE2 production preincubated for 10 mins followed by substrate ... | Bioorg Med Chem Lett 26: 1411-5 (2016) BindingDB Entry DOI: 10.7270/Q2X92D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate assessed as PGE2 production preincubated for 10 mins followed by substrate addition inc... | Bioorg Med Chem Lett 26: 1411-5 (2016) BindingDB Entry DOI: 10.7270/Q2X92D52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Antiproliferative activity against MDR human HL60R cell line | J Med Chem 49: 3012-8 (2006) Article DOI: 10.1021/jm060253o BindingDB Entry DOI: 10.7270/Q2R49RKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcr-Abl (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Antiproliferative activity against human K562 cell line expressing Bcr-Abl | J Med Chem 49: 3012-8 (2006) Article DOI: 10.1021/jm060253o BindingDB Entry DOI: 10.7270/Q2R49RKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate | Bioorg Med Chem 21: 6022-37 (2013) Article DOI: 10.1016/j.bmc.2013.07.037 BindingDB Entry DOI: 10.7270/Q21R6RX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania "L. Vanvitelli" Curated by ChEMBL | Assay Description Inhibition of human 5-LOX expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins followed by substrate addit... | Eur J Med Chem 180: 637-647 (2019) Article DOI: 10.1016/j.ejmech.2019.07.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1B1 | J Med Chem 45: 160-4 (2001) BindingDB Entry DOI: 10.7270/Q2668CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1A2 | J Med Chem 45: 160-4 (2001) BindingDB Entry DOI: 10.7270/Q2668CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.56E+6 | n/a | n/a | n/a | n/a |
University of Houston Curated by ChEMBL | Assay Description Binding affinity to recombinant C1 domain of PKCalpha expressed in Escherichia coli BL21 (DE3) after 45 mins by fluorescence quenching assay | Bioorg Med Chem 19: 5321-33 (2011) Article DOI: 10.1016/j.bmc.2011.08.008 BindingDB Entry DOI: 10.7270/Q25H7GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM23928 (1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Antagonist activity at rat TRPA1 expressed in HEK293 cells assessed as inhibition of allyl isothiocyanate-induced increase of intracellular calcium l... | Bioorg Med Chem Lett 26: 899-902 (2016) BindingDB Entry DOI: 10.7270/Q2765H6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||