

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)N(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12)[C@H]1C[C@H](CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific [1-416] (Homo sapiens (Human)) | BDBM244794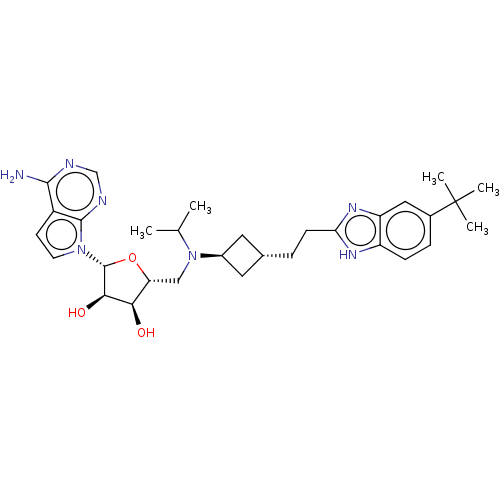 (US9446064, A75) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description An in vitro biological assay that can be used includes the steps of (1) mixing a histone substrate (e.g., an isolated histone sample for a histone or... | US Patent US9446064 (2016) BindingDB Entry DOI: 10.7270/Q2QZ28WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||