

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM25008 4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,5-c]pyridin-4-yl]-2-methylbut-3-yn-2-ol::GSK screening, 27::oxadiazole-containing compound, 3b
SMILES: CCn1c(nc2c(ncc(OCC3CCNCC3)c12)C#CC(C)(C)O)-c1nonc1N
InChI Key: InChIKey=NTPAYIOIMIUCMN-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25008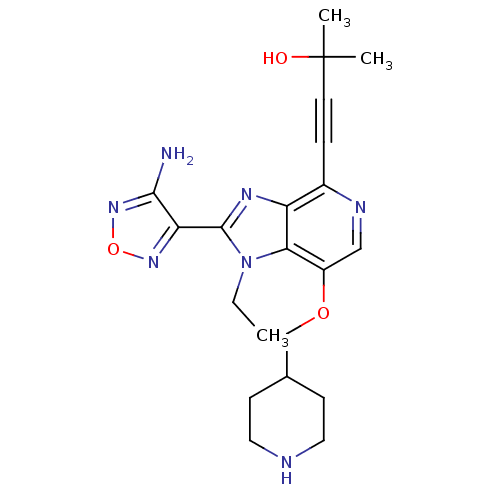 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein cereblon/Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM25008 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 718 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund | Assay Description IC50 determinations for TBK1 were performed with the KinEASE-STK assay from Cisbio according to the manufacturer's instructions. A biotinylated s... | ACS Chem Biol 10: 289-98 (2015) Article DOI: 10.1021/cb500908d BindingDB Entry DOI: 10.7270/Q2WH2NRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT (Homo sapiens (Human)) | BDBM25008 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT2 (Homo sapiens (Human)) | BDBM25008 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||