 Found 6 hits for monomerid = 25014
Found 6 hits for monomerid = 25014 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25014
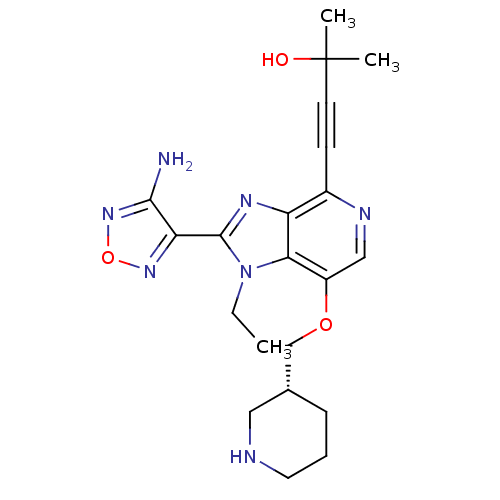
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25014

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 |
J Med Chem 53: 1413-37 (2010)
Article DOI: 10.1021/jm901132v
BindingDB Entry DOI: 10.7270/Q2VD6ZK0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM25014

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 |
J Med Chem 53: 1413-37 (2010)
Article DOI: 10.1021/jm901132v
BindingDB Entry DOI: 10.7270/Q2VD6ZK0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25014

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 |
J Med Chem 53: 1413-37 (2010)
Article DOI: 10.1021/jm901132v
BindingDB Entry DOI: 10.7270/Q2VD6ZK0 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM25014

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 |
J Med Chem 53: 1413-37 (2010)
Article DOI: 10.1021/jm901132v
BindingDB Entry DOI: 10.7270/Q2VD6ZK0 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25014

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 |
J Med Chem 53: 1413-37 (2010)
Article DOI: 10.1021/jm901132v
BindingDB Entry DOI: 10.7270/Q2VD6ZK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data