 Found 18 hits for monomerid = 25036
Found 18 hits for monomerid = 25036 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25036
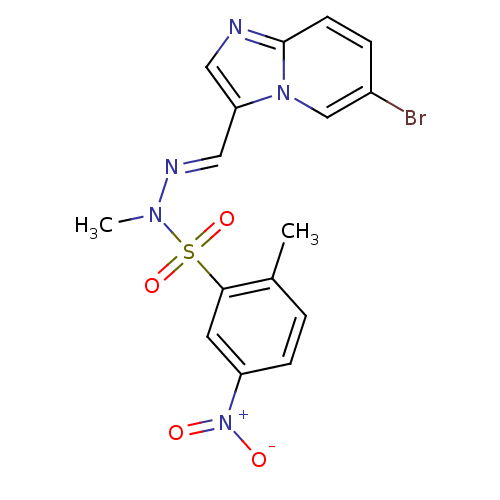
(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM
US Patent
| Assay Description
MELK and its substrate, Bcl-G were both recombinantly expressed and purified for use in screening assays (See Methods). 752 compounds from an in-hous... |
US Patent US10981896 (2021)
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Glycogen Synthase Kinase 3 (GSK3)
(Ustilago maydis (Smut fungus)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Dortmund
| Assay Description
Inhibition of UmGSK3 by kinase inhibitors. |
ACS Chem Biol 7: 1257-67 (2012)
Article DOI: 10.1021/cb300128b
BindingDB Entry DOI: 10.7270/Q29S1PNH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Dortmund
| Assay Description
Inhibition of UmGSK3 by kinase inhibitors. |
ACS Chem Biol 7: 1257-67 (2012)
Article DOI: 10.1021/cb300128b
BindingDB Entry DOI: 10.7270/Q29S1PNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p110delta |
Bioorg Med Chem 15: 7677-87 (2007)
Article DOI: 10.1016/j.bmc.2007.08.062
BindingDB Entry DOI: 10.7270/Q23X86BV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p110alpha |
Bioorg Med Chem 15: 7677-87 (2007)
Article DOI: 10.1016/j.bmc.2007.08.062
BindingDB Entry DOI: 10.7270/Q23X86BV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p110beta |
Bioorg Med Chem 15: 7677-87 (2007)
Article DOI: 10.1016/j.bmc.2007.08.062
BindingDB Entry DOI: 10.7270/Q23X86BV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Melbourne
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by radiometric assay |
Bioorg Med Chem 23: 6280-96 (2015)
BindingDB Entry DOI: 10.7270/Q2KK9DM6 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Melbourne
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) using biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 as substrate after 90 mins b... |
Bioorg Med Chem 23: 6280-96 (2015)
BindingDB Entry DOI: 10.7270/Q2KK9DM6 |
More data for this
Ligand-Target Pair | |
Cyclin-Dependent Kinase 9 (CDK9)
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Melbourne
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK9/cyclin-T1 using H-YSPTSPSYSPTSPSYSPTSPS-KKKK-OH as substrate after 90 mins by luminescence assay |
Bioorg Med Chem 23: 6280-96 (2015)
BindingDB Entry DOI: 10.7270/Q2KK9DM6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to full length human CDK9 (1 to 372 residues) expressed in bacterial expression system by KINOMEscan competition assay |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to full length human CDK7 (1 to 344 residues) expressed in mammalian expression system by KINOMEscan competition assay |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK9 (unknown origin) |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to full length human CDK2 (1 to 298 residues) expressed in bacterial expression system by KINOMEscan competition assay |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25036

(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data