

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM251922 US10065961, Compound 22::US10683295, Compound 22::US10941151, Compound 22::US9475814, 22
SMILES: C[C@H]1N(CCn2c1nnc2-c1nc(C)c(C)s1)C(=O)c1ccc(cc1)-c1cccs1
InChI Key: InChIKey=KSIGHAFTVLYICN-CQSZACIVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251922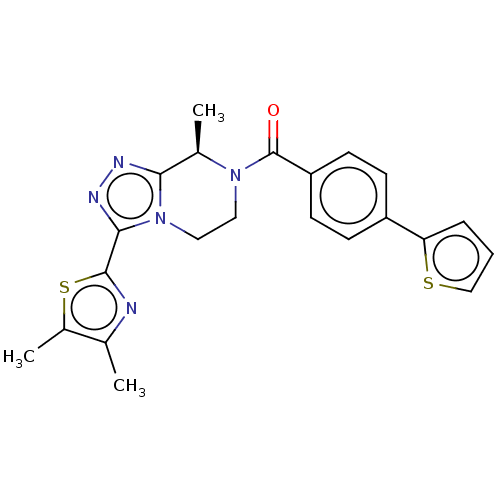 (US10065961, Compound 22 | US10683295, Compound 22 ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 3 receptor (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 3 receptor (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2/HERG (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description hERG: The hERG inhibition study aims at quantifying the in vitro effects of compounds of the invention on the potassium-selective IKr current generat... | US Patent US10683295 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen S.A. US Patent | Assay Description The hERG inhibition study aims at quantifying the in vitro effects of compounds of the invention on the potassium-selective IKf current generated in ... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen S.A. US Patent | Assay Description Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional regulator ERG (Homo sapiens (Human)) | BDBM251922 (US10065961, Compound 22 | US10683295, Compound 22 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The human Ether-a-go-go Related Gene (hERG) encodes the inward rectifying voltage gated potassium channel in the heart (IKr) which is involved in car... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||