

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM25269 (1-hydroxy-1-phosphonodecyl)phosphonic acid::CHEMBL54004::bisphosphonate, 23
SMILES: CCCCCCCCCC(O)(P(O)(O)=O)P(O)(O)=O
InChI Key: InChIKey=NSCPCMXKWUFFNL-UHFFFAOYSA-N
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesyl diphosphate synthase (Homo sapiens (Human)) | BDBM25269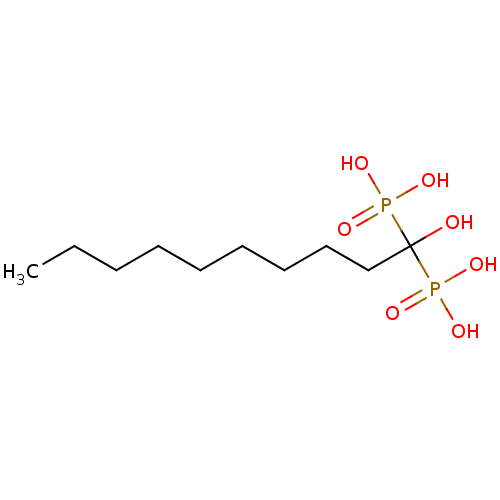 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl diphosphate synthase (Homo sapiens (Human)) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against farnesyl Pyrophosphate Synthase was determined | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Geranylgeranyl Diphosphate Synthase (GGPPS) (Homo sapiens (Human)) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against the human recombinant geranylgeranyl diphosphate synthase (GGPPSase). | J Med Chem 45: 2185-96 (2002) BindingDB Entry DOI: 10.7270/Q2KH0MN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase (Trypanosoma cruzi) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Venezolano de Investigaciones Cient£ficas Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi hexokinase | J Biol Chem 282: 12377-87 (2007) Article DOI: 10.1074/jbc.M607286200 BindingDB Entry DOI: 10.7270/Q2FT8MXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase (Trypanosoma cruzi) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi hexokinase | J Med Chem 49: 215-23 (2006) Article DOI: 10.1021/jm0582625 BindingDB Entry DOI: 10.7270/Q2BV7G6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Plasmodium falciparum (isolate 3D7)) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against FPPS in Leishmania major | J Med Chem 49: 215-23 (2006) Article DOI: 10.1021/jm0582625 BindingDB Entry DOI: 10.7270/Q2BV7G6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vacuolar-type proton translocating pyrophosphatase 1 (Trypanosoma brucei) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma brucei soluble vacuolar pyrophosphatase expressed in Escherichia coli | J Med Chem 48: 6128-39 (2005) Article DOI: 10.1021/jm058220g BindingDB Entry DOI: 10.7270/Q2F47PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl diphosphate synthase (Homo sapiens (Human)) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M) | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Geranylgeranyl Diphosphate Synthase (GGPPS) (Homo sapiens (Human)) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Plasmodium falciparum (isolate 3D7)) | BDBM25269 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||