 Found 13 hits for monomerid = 25298
Found 13 hits for monomerid = 25298 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298
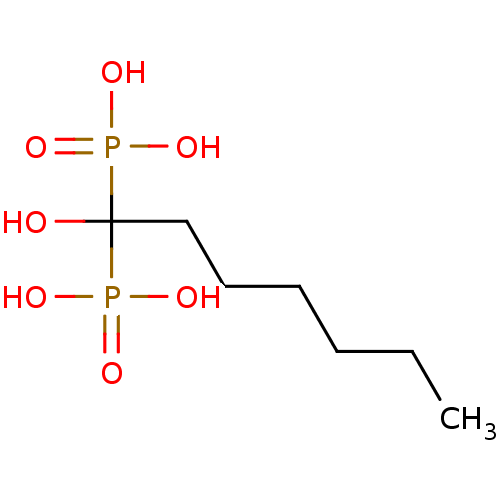
((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Trypanosoma brucei farnesyl pyrophosphate synthase activity |
Bioorg Med Chem Lett 13: 3231-5 (2003)
BindingDB Entry DOI: 10.7270/Q2ZK5G2R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl diphosphate synthase
(Trypanosoma cruzi) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi FPPS |
Bioorg Med Chem 16: 3283-90 (2008)
Article DOI: 10.1016/j.bmc.2007.12.010
BindingDB Entry DOI: 10.7270/Q2JD4XPC |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi farnesyl pyrophosphate synthase (TcFPPS) |
Bioorg Med Chem Lett 15: 4685-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.060
BindingDB Entry DOI: 10.7270/Q21Z43ZW |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Plasmodium falciparum (isolate 3D7)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leishmania major Farnesyl diphosphate synthase |
J Med Chem 47: 175-87 (2003)
Article DOI: 10.1021/jm030084x
BindingDB Entry DOI: 10.7270/Q2FN15N5 |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Trypanosoma cruzi) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi FPPS |
Bioorg Med Chem 16: 3283-90 (2008)
Article DOI: 10.1016/j.bmc.2007.12.010
BindingDB Entry DOI: 10.7270/Q2JD4XPC |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Trypanosoma brucei farnesyl pyrophosphate synthase activity |
Bioorg Med Chem Lett 13: 3231-5 (2003)
BindingDB Entry DOI: 10.7270/Q2ZK5G2R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M) |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi farnesyl pyrophosphate synthase |
Bioorg Med Chem Lett 13: 3231-5 (2003)
BindingDB Entry DOI: 10.7270/Q2ZK5G2R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Geranylgeranyl Diphosphate Synthase (GGPPS)
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against the human recombinant geranylgeranyl diphosphate synthase (GGPPSase). |
J Med Chem 45: 2185-96 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0MN1 |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl Pyrophosphate Synthase was determined |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Geranylgeranyl Diphosphate Synthase (GGPPS)
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM25298

((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibitory concentration against trypanosoma cruzi farnesyl pyrophosphate synthase (TcFPPS) |
Bioorg Med Chem Lett 15: 4685-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.060
BindingDB Entry DOI: 10.7270/Q21Z43ZW |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data