

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM25911 3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole-5-sulfonamide::BMC173212 Compound 2g::indole sulfonamide, 8g
SMILES: NNC(=O)c1[nH]c2ccc(cc2c1-c1ccccc1Cl)S(N)(=O)=O
InChI Key: InChIKey=POTAERZUCKEKBH-UHFFFAOYSA-N
Data: 15 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic Anhydrase XIV (Homo sapiens (Human)) | BDBM25911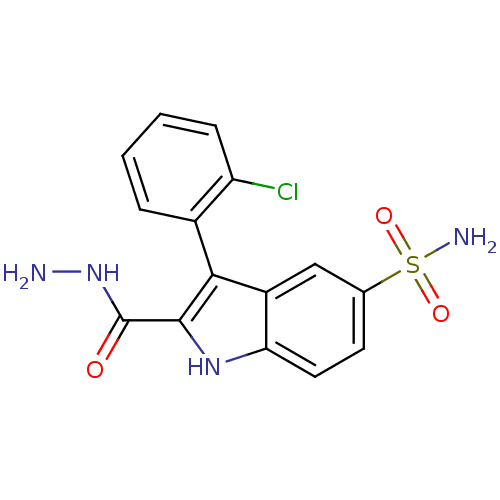 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38.8 | -10.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| beta-Carbonic Anhydrase (Candida albicans (Yeast)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description inhibition of Candida albicans recombinant Nce103 after 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem Lett 20: 2508-11 (2010) Article DOI: 10.1016/j.bmcl.2010.02.103 BindingDB Entry DOI: 10.7270/Q23F4PS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XV (Mus musculus (mouse)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | KEGG GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-Carbonic Anhydrase (Caenorhabditis elegans) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 372 | -8.77 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Istanbul University | Assay Description An SX.18MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic/inhibition of various CA isozymes. Phenol... | Bioorg Med Chem 17: 3212-5 (2009) Article DOI: 10.1016/j.bmc.2009.01.048 BindingDB Entry DOI: 10.7270/Q2MS3R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 640 | -8.44 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase VA (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 855 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIII (Mus musculus (mouse)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 935 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase VB (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 937 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (CA-VI) (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase III (Homo sapiens (Human)) | BDBM25911 (3-(2-chlorophenyl)-2-(hydrazinecarbonyl)-1H-indole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 9113-20 (2008) Article DOI: 10.1016/j.bmc.2008.09.032 BindingDB Entry DOI: 10.7270/Q2ZW1J7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||