

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM2600 (Arylindolyl)maleimide deriv. 22::3-(1-benzofuran-3-yl)-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione::CHEMBL301138
SMILES: Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2ccccc12
InChI Key: InChIKey=KCBBKYDLIADLOS-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Kinase C (Rattus norvegicus (rat)) | BDBM2600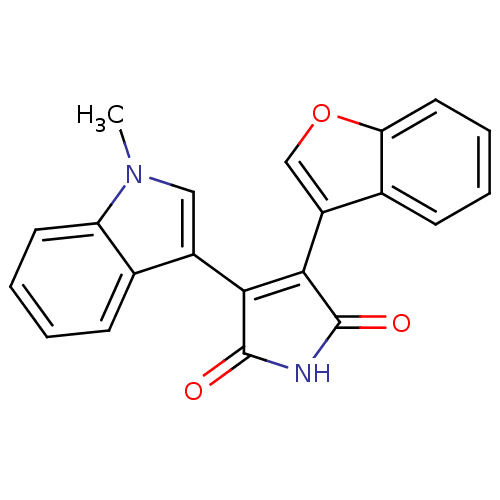 ((Arylindolyl)maleimide deriv. 22 | 3-(1-benzofuran...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM2600 ((Arylindolyl)maleimide deriv. 22 | 3-(1-benzofuran...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM2600 ((Arylindolyl)maleimide deriv. 22 | 3-(1-benzofuran...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||