

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM2604 (Arylindolyl)maleimide deriv. 26::3-(1-methyl-1H-indol-3-yl)-4-(thiophen-2-yl)-2,5-dihydro-1H-pyrrole-2,5-dione
SMILES: Cn1cc(C2=C(C(=O)NC2=O)c2cccs2)c2ccccc12
InChI Key: InChIKey=SHHFWYXUKQGJFE-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Kinase C (Rattus norvegicus (rat)) | BDBM2604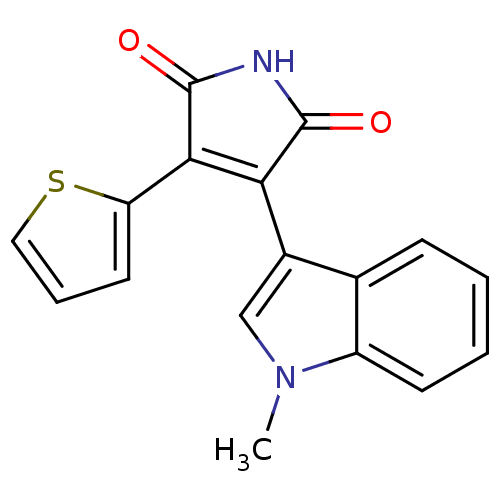 ((Arylindolyl)maleimide deriv. 26 | 3-(1-methyl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 35: 177-84 (1992) Article DOI: 10.1021/jm00079a024 BindingDB Entry DOI: 10.7270/Q2K64G8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM2604 ((Arylindolyl)maleimide deriv. 26 | 3-(1-methyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM2604 ((Arylindolyl)maleimide deriv. 26 | 3-(1-methyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of PKCalpha | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||