

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26195 4-aminophenol::phenol derivative, 9
SMILES: Nc1ccc(O)cc1
InChI Key: InChIKey=PLIKAWJENQZMHA-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer. 195 PDB IDs contain this monomer as substructures. 195 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic Anhydrase III (Homo sapiens (Human)) | BDBM26195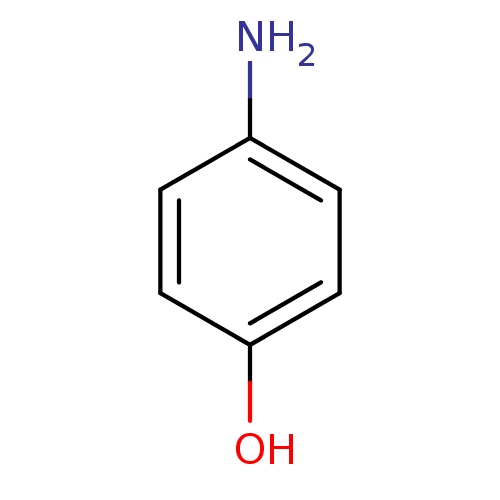 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIV (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (CA-VI) (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.59E+5 | -5.18 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 18: 3593-6 (2008) Article DOI: 10.1016/j.bmcl.2008.04.077 BindingDB Entry DOI: 10.7270/Q2XP75TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 15 (Mus musculus) | BDBM26195 (4-aminophenol | phenol derivative, 9) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of mouse recombinant carbonic anhydrase 15 by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 18: 3593-6 (2008) Article DOI: 10.1016/j.bmcl.2008.04.077 BindingDB Entry DOI: 10.7270/Q2XP75TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase VB (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase XIII (Mus musculus (mouse)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.52E+5 | -4.26 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 18: 3593-6 (2008) Article DOI: 10.1016/j.bmcl.2008.04.077 BindingDB Entry DOI: 10.7270/Q2XP75TC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic Anhydrase VA (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem 16: 7424-8 (2008) Article DOI: 10.1016/j.bmc.2008.06.013 BindingDB Entry DOI: 10.7270/Q2X065C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of KAT3B catalytic domain (1284 to 1673 residues) (unknown origin) using SGRGKGGKGLGKGGAKRHRK-NH2 as substrate after 5 mins in presence of... | Eur J Med Chem 136: 480-486 (2017) Article DOI: 10.1016/j.ejmech.2017.05.015 BindingDB Entry DOI: 10.7270/Q20Z75S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2A/KAT2B (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of KAT2B catalytic domain (492 to 658 residues) (unknown origin) using H-ARTKQTARKSTGGKAPRKQL-OH as substrate after 5 mins in presence of ... | Eur J Med Chem 136: 480-486 (2017) Article DOI: 10.1016/j.ejmech.2017.05.015 BindingDB Entry DOI: 10.7270/Q20Z75S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT8 (Homo sapiens (Human)) | BDBM26195 (4-aminophenol | phenol derivative, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Tested for antagonist activity against NK-3 receptor in rat portal vein by using Neurokinin B as agonist | Eur J Med Chem 136: 480-486 (2017) Article DOI: 10.1016/j.ejmech.2017.05.015 BindingDB Entry DOI: 10.7270/Q20Z75S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||