

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26261 (2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide::C-31::CHEMBL512283::US9278914, S-II
SMILES: C[C@](O)(COc1ccc(Cl)c(F)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F
InChI Key: InChIKey=SSFVOEAXHZGTRJ-KRWDZBQOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen Receptor (Homo sapiens (Human)) | BDBM26261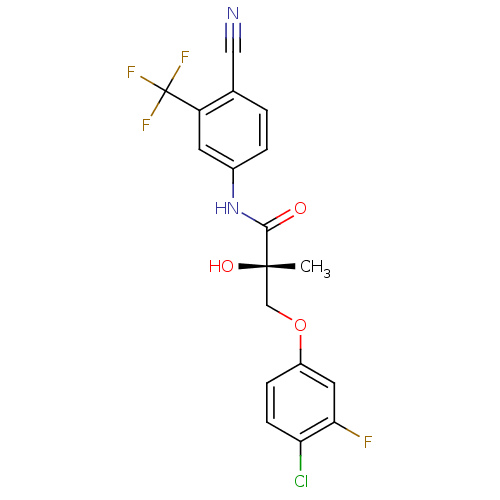 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | -11.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tennessee Research Foundation US Patent | Assay Description P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar... | US Patent US9278914 (2016) BindingDB Entry DOI: 10.7270/Q2D21WF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tennessee Research Foundation US Patent | Assay Description P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar... | US Patent US9278914 (2016) BindingDB Entry DOI: 10.7270/Q2D21WF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tennessee Research Foundation US Patent | Assay Description P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar... | US Patent US9278914 (2016) BindingDB Entry DOI: 10.7270/Q2D21WF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tennessee Research Foundation US Patent | Assay Description P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar... | US Patent US9278914 (2016) BindingDB Entry DOI: 10.7270/Q2D21WF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM26261 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tennessee Research Foundation US Patent | Assay Description P450 enzyme inhibition was measured using human cDNA-expressed CYP3A4, 2D6, 2C19, 2C9, and 1A2 recombinant enzymes and fluorogenic substrates (coumar... | US Patent US9278914 (2016) BindingDB Entry DOI: 10.7270/Q2D21WF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||