

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM263393 US9550741, II-3
SMILES: O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccco1
InChI Key: InChIKey=PPOMMOPYGUOVFS-WGSAOQKQSA-N
Data: 11 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263393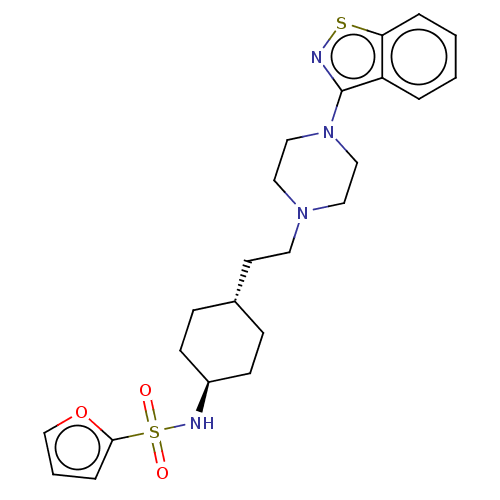 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description The experiment is carried out by the method according to Journal of Pharmacology and Experimental Therapeutics 2010, 333(1): 328. With [3H]methyl-spi... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | 7.7 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials(1) 5-HT2A Cell Transfection:This experiment utilizes the plasmid vector containing the gene of the 5-HT2A receptor protein ... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | 7.7 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials(1) 5-HT2A Cell Transfection:This experiment utilizes the plasmid vector containing the gene of the 5-HT2A receptor protein ... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | 7.7 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials(1) 5-HT2A Cell Transfection:This experiment utilizes the plasmid vector containing the gene of the 5-HT2A receptor protein ... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | 7.7 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials(1) 5-HT2A Cell Transfection:This experiment utilizes the plasmid vector containing the gene of the 5-HT2A receptor protein ... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials:(1) D2 receptor Cell Transfection:This experiment utilizes the plasmid vector containing the gene of D2 receptor protein fo... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.98 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials:(1) D2 receptor Cell Transfection:This experiment utilizes the plasmid vector containing the gene of D2 receptor protein fo... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials:The isotopic ligand of 5-H1A receptor [3H]0.8-OH-DPAT (purchased from PE Corporation), (+)5-hydroxytryptamine (purchased fr... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials:The isotopic ligand of 5-H1A receptor [3H]0.8-OH-DPAT (purchased from PE Corporation), (+)5-hydroxytryptamine (purchased fr... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM263393 (US9550741, II-3) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.72 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Shanghai Institute of Pharmaceutical Industry US Patent | Assay Description 1. Experimental Materials:The isotopic ligand of 5-H1A receptor [3H]0.8-OH-DPAT (purchased from PE Corporation), (+)5-hydroxytryptamine (purchased fr... | US Patent US9550741 (2017) BindingDB Entry DOI: 10.7270/Q2DN4726 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||