

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OC(=O)CC[C@@H](NS(=O)(=O)c1ccc2cc(OCCCc3ccccc3)ccc2c1)C(O)=O
InChI Key: InChIKey=HYGXSIARDUDLTA-JOCHJYFZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-N-acetylmuramoylalanine--D-glutamate ligase (Escherichia coli (strain K12)) | BDBM26452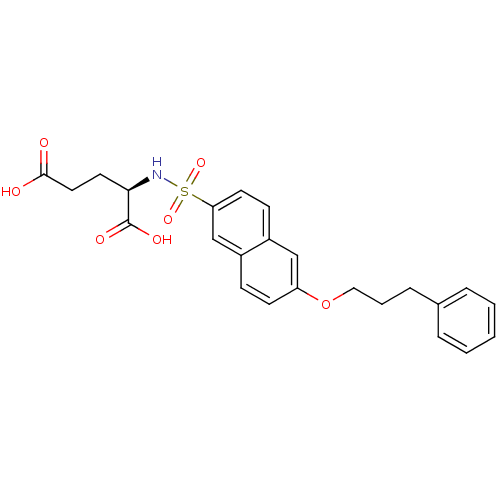 ((2R)-2-{[6-(3-phenylpropoxy)naphthalene-2-]sulfona...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | 8.6 | 37 |
Lek Pharmaceuticals d.d. | Assay Description The compounds were tested for their ability to inhibit the addition of D-[14C]Glu to UMA. The radioactive substrate and product were separated by re... | J Med Chem 51: 7486-94 (2008) Article DOI: 10.1021/jm800762u BindingDB Entry DOI: 10.7270/Q2B27SMN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||