

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26613 (2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-enoic acid::Compound 13778
SMILES: OC(=O)\C=C\c1cccc(c1)[Sb](O)(O)=O
InChI Key: InChIKey=ZTQRMGDLUGQTTF-MNPOOLNOSA-L
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| phosphatase Cdc25 (Homo sapiens (Human)) | BDBM26613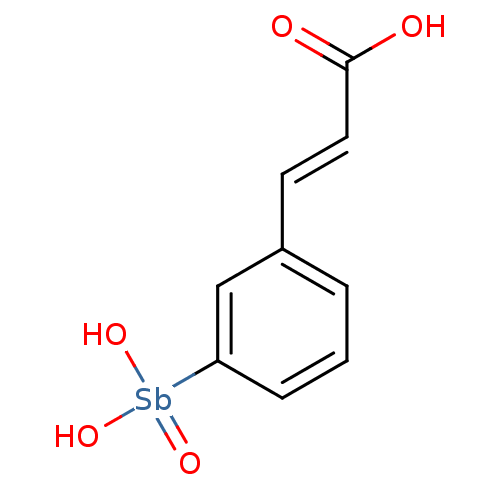 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Cdc25a catalytic domain expressed in Escherichia coli BL21(DE3) using 3-O-Methylfluorescein phosphate as substrat... | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity phosphatase Cdc25B (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Cdc25b catalytic domain expressed in Escherichia coli BL21(DE3) using 3-O-Methylfluorescein phosphate as substrat... | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged VHR using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition b... | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (PTPβ) (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of PTP-beta receptor using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 6 (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of MKP3 using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of PTP1B using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylglycerophosphatase and protein-tyrosine phosphatase 1 (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of PTPMT1 using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (PTPβ) (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of PTP-beta receptor using para-nitrophenol as substrate by colorimetric analysis | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| phosphatase Cdc25 (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Cdc25a catalytic domain expressed in Escherichia coli BL21(DE3) using 3-O-Methylfluorescein phosphate as substrat... | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity phosphatase Cdc25B (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Cdc25b catalytic domain expressed in Escherichia coli BL21(DE3) using 3-O-Methylfluorescein phosphate as substrat... | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cdc25C (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged Cdc25c catalytic domain expressed in Escherichia coli BL21(DE3) using 3-O-Methylfluorescein phosphate as substrat... | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apurinic-apyrimidinic endonuclease 1 (APE-1) (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Johns Hopkins University | Assay Description The screening assay was performed using 384-well microtiter plate first spotted with test compounds. Then Ape1 in reaction buffer was added to each w... | Mol Pharmacol 73: 669-77 (2008) Article DOI: 10.1124/mol.107.042622 BindingDB Entry DOI: 10.7270/Q2WS8RJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (Homo sapiens (Human)) | BDBM26613 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human PTEN using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||