

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26656 5,7,3,4,5-pentahydroxyflavone::5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one
SMILES: Oc1cc(O)c2c(c1)oc(cc2=O)-c1cc(O)c(O)c(O)c1
InChI Key: InChIKey=ARSRJFRKVXALTF-UHFFFAOYSA-N
Data: 5 IC50
PDB links: 1 PDB ID matches this monomer. 4 PDB IDs contain this monomer as substructures. 4 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM26656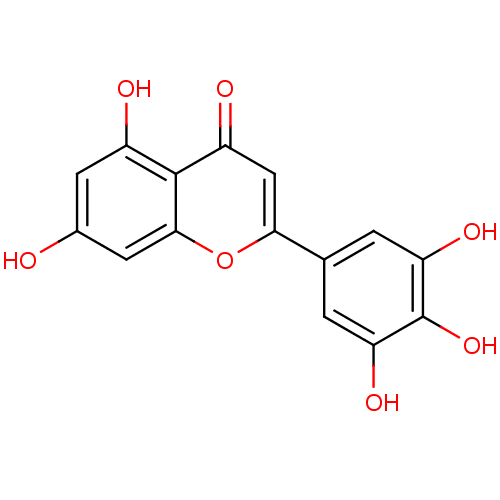 (5,7,3,4,5-pentahydroxyflavone | 5,7-dihydroxy-2-(3...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Loma Linda University | Assay Description The 96-well flat-bottomed plates were coated with recombinant GST-BAD. After the plates were blocked, the reaction buffer containing test compound an... | Mol Cancer Ther 6: 163-72 (2007) Article DOI: 10.1158/1535-7163.MCT-06-0397 BindingDB Entry DOI: 10.7270/Q2N8783K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM26656 (5,7,3,4,5-pentahydroxyflavone | 5,7-dihydroxy-2-(3...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
Loma Linda University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase | Bioorg Med Chem 15: 6463-73 (2007) Checked by Author Article DOI: 10.1016/j.bmc.2007.06.025 BindingDB Entry DOI: 10.7270/Q2C24W4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM26656 (5,7,3,4,5-pentahydroxyflavone | 5,7-dihydroxy-2-(3...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant PIM1 (unknown origin) expressed in Escherichia coli after 60 mins by ELISA | Bioorg Med Chem 27: 677-685 (2019) Article DOI: 10.1016/j.bmc.2019.01.027 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase S (Homo sapiens (Human)) | BDBM26656 (5,7,3,4,5-pentahydroxyflavone | 5,7-dihydroxy-2-(3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP-sigma (residues 1367 to 1948) using para-nitrophenylphosphate as substrate for 60 mins by fluorescence analysis | Bioorg Med Chem Lett 26: 87-93 (2016) Article DOI: 10.1016/j.bmcl.2015.11.026 BindingDB Entry DOI: 10.7270/Q2M048FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase 2 (Homo sapiens (Human)) | BDBM26656 (5,7,3,4,5-pentahydroxyflavone | 5,7-dihydroxy-2-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Neu2 assessed as MuNANA substrate hydrolysis in presence of 0.1% Triton X-100 by discontinuous fluorimetric assay | Bioorg Med Chem 18: 1633-40 (2010) Article DOI: 10.1016/j.bmc.2009.12.062 BindingDB Entry DOI: 10.7270/Q26110F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||