

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26808 (7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl)methoxy]benzene}-1,4-dioxaspiro[4.4]nonane-7,8-diamido::1,3-dioxolane beta-amino hydroxamate , 1
SMILES: Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1
InChI Key: InChIKey=KRGIXMSVPSMLII-JTHBVZDNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26808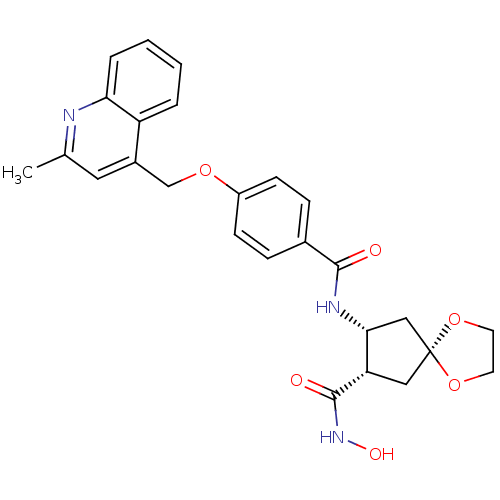 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.13E+3 | >-7.73 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP9 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -7.57 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP2 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP1 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-7.23 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADAM17 (Sus scrofa (pig)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of pig TACE | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADAM17 (Sus scrofa (pig)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||