

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1(C)CCC(N2CCC3(CC2)N(CN(CCNCc2ccccc2)C3=O)c2ccccc2)c2ccccc12
InChI Key: InChIKey=GZSRIFUGJZDLKC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26951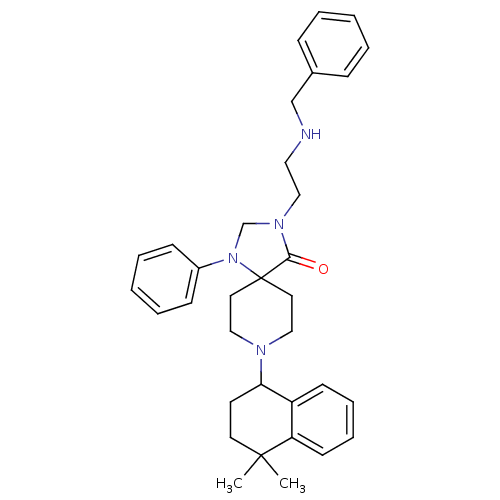 (3-[2-(benzylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM26951 (3-[2-(benzylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM26951 (3-[2-(benzylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM26951 (3-[2-(benzylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||