

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM27002 2-[(3S,14S,17S,18R,20S)-3-[(tert-butylcarbamoyl)amino]-19,19-dimethyl-2,16-dioxo-1,15-diazatricyclo[15.4.0.0^{18,20}]henicosan-14-yl]-N-({[(S)-(dimethylcarbamoyl)(phenyl)methyl]carbamoyl}methyl)-2-oxoacetamide::ketoamide derived macrocyclic inhibitor, 26
SMILES: CN(C)C(=O)[C@@H](NC(=O)CNC(=O)C(=O)[C@@H]1CCCCCCCCCC[C@H](NC(=O)NC(C)(C)C)C(=O)N2C[C@H]3[C@@H]([C@H]2C(=O)N1)C3(C)C)c1ccccc1
InChI Key: InChIKey=MFLYVBSEKPNJNJ-MVTDWJHSSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCV NS3-NS4A Serine Proteinase (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM27002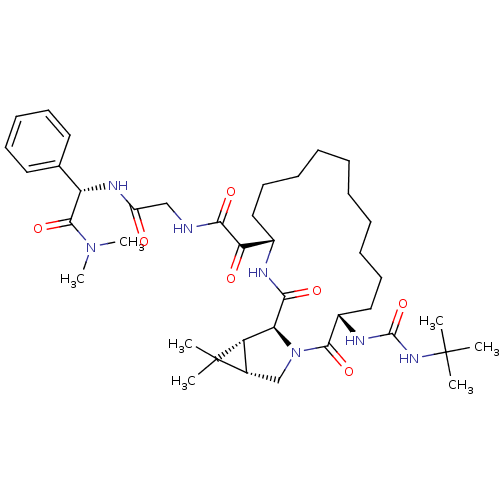 (2-[(3S,14S,17S,18R,20S)-3-[(tert-butylcarbamoyl)am...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | -11.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 52: 336-46 (2009) Article DOI: 10.1021/jm800940u BindingDB Entry DOI: 10.7270/Q2639N17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||