

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM2716 1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,6-difluorophenyl]ethyl}urea::CHEMBL39665::N-[2-(2,6-Difluoro-3-(dimethylamino)phenethyl)]-N-[2-(5-bromopyridyl)]urea::urea-PETT deriv. 1
SMILES: CN(C)c1ccc(F)c(CCNC(=O)Nc2ccc(Br)cn2)c1F
InChI Key: InChIKey=XGBUEAAOXNGUJL-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Reverse Transcriptase (Human immunodeficiency virus type 1) | BDBM2716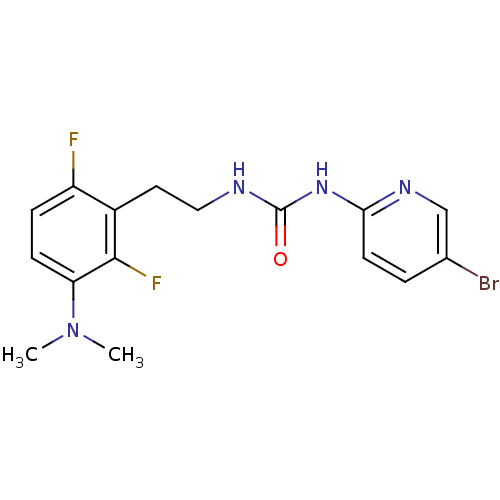 (1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Medivir AB | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 4150-60 (1999) Article DOI: 10.1021/jm990095j BindingDB Entry DOI: 10.7270/Q25X273G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Reverse Transcriptase Mutant (Y181C) (Human immunodeficiency virus type 1) | BDBM2716 (1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Medivir AB | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 4150-60 (1999) Article DOI: 10.1021/jm990095j BindingDB Entry DOI: 10.7270/Q25X273G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM2716 (1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibitory effect on wild type HIV- 1 reverse transcriptase using rCdG as template and dGTP as substrate. | Bioorg Med Chem Lett 8: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2RR1XDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM2716 (1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibitory effect on recombinant HIV- 1 reverse transcriptase which has a mutation Leu 100 to Ile 100 (clone 118) | Bioorg Med Chem Lett 8: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2RR1XDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM2716 (1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibitory effect on wild type HIV- 1 reverse transcriptase using rCdG as template and dGTP as substrate. | Bioorg Med Chem Lett 8: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2RR1XDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Reverse Transcriptase Mutant (K103N) (Human immunodeficiency virus type 1) | BDBM2716 (1-(5-bromopyridin-2-yl)-3-{2-[3-(dimethylamino)-2,...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Medivir AB | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 4150-60 (1999) Article DOI: 10.1021/jm990095j BindingDB Entry DOI: 10.7270/Q25X273G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||