

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM27351 2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyridin-4-one::Pyrrolopyridinone, 5
SMILES: Nc1nccc(n1)-c1cc2c(CCNC2=O)[nH]1
InChI Key: InChIKey=FMVMPEYKBFJPAD-UHFFFAOYSA-N
Data: 5 IC50
PDB links: 1 PDB ID contains this monomer as substructures. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cdc7 Kinase (Homo sapiens (Human)) | BDBM27351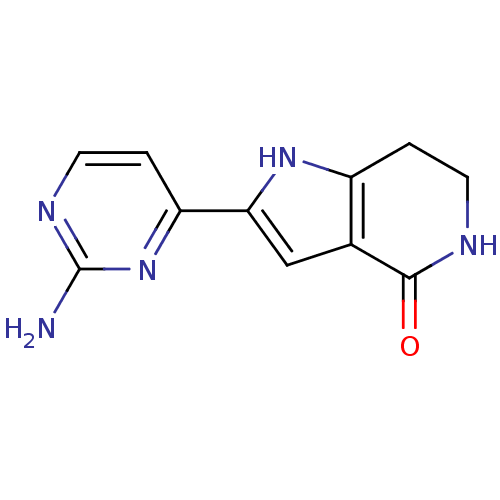 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cdc7 Kinase (Homo sapiens (Human)) | BDBM27351 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27351 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of Aurora-A | Bioorg Med Chem Lett 22: 96-101 (2011) Article DOI: 10.1016/j.bmcl.2011.11.065 BindingDB Entry DOI: 10.7270/Q2W959NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM27351 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of PLK1 | Bioorg Med Chem Lett 22: 96-101 (2011) Article DOI: 10.1016/j.bmcl.2011.11.065 BindingDB Entry DOI: 10.7270/Q2W959NX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| CDK2/Cyclin A/Cyclin A1 (Homo sapiens (Human)) | BDBM27351 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A | Bioorg Med Chem Lett 22: 96-101 (2011) Article DOI: 10.1016/j.bmcl.2011.11.065 BindingDB Entry DOI: 10.7270/Q2W959NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||