

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM27391 5,13,17-triazatetracyclo[8.7.0.0^{2,7}.0^{11,16}]heptadeca-1(10),2(7),3,5,8,11(16)-hexaen-12-one::pyrrolopyridinone scaffold, 13
SMILES: O=C1NCCc2[nH]c3c(ccc4cnccc34)c12
InChI Key: InChIKey=QWQJKPPUDAAQOJ-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cdc7 Kinase (Homo sapiens (Human)) | BDBM27391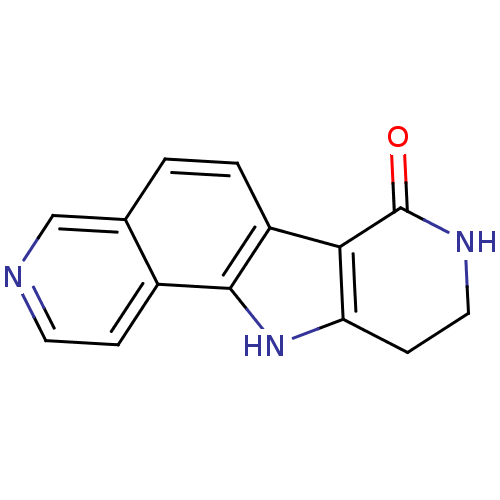 (5,13,17-triazatetracyclo[8.7.0.0^{2,7}.0^{11,16}]h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||