

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM276092 5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl-4-{1H-[1,2,3]triazolo[4,5-c]pyridin-6-yl}-1H-imidazole::US10071984, Example 54::US10174003, Example 54::US9896436, Example 54
SMILES: Cn1cnc(c1-c1ccc(F)cc1OCC1CC1)-c1cc2[nH]nnc2cn1
InChI Key: InChIKey=IJEOCHNJDZZTLK-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 2B (Homo sapiens (Human)) | BDBM276092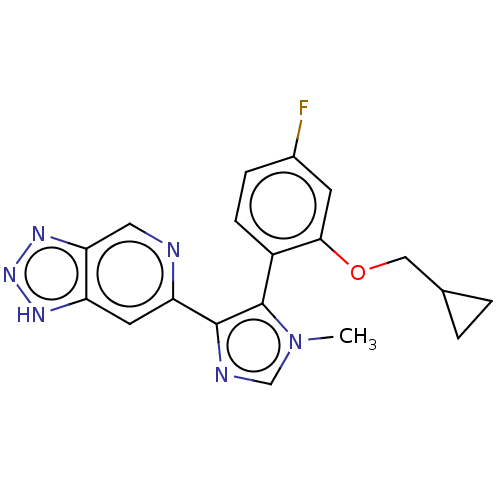 (5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | 25 |
Celgene Quanticel Research, Inc. US Patent | Assay Description The ability of test compounds to inhibit the activity of FBXL10 was determined in 384-well plate format under the following reaction conditions: 0.3 ... | US Patent US10071984 (2018) BindingDB Entry DOI: 10.7270/Q24Q7X0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone lysine demethylase PHF8 (2) (Homo sapiens (Human)) | BDBM276092 (5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Celgene Quanticel Research, Inc. US Patent | Assay Description The ability of test compounds to inhibit the activity of PHF8 was determined in 384-well plate format under the following reaction conditions: 3 nM P... | US Patent US10071984 (2018) BindingDB Entry DOI: 10.7270/Q24Q7X0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone lysine demethylase PHF8 (Homo sapiens (Human)) | BDBM276092 (5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description PHF8 Assay: The ability of test compounds to inhibit the activity of PHF8 was determined in 384-well plate format under the following reaction condit... | Bioorg Med Chem Lett 17: 1584-9 (2007) BindingDB Entry DOI: 10.7270/Q2FT8PBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone lysine demethylase PHF8 (Homo sapiens (Human)) | BDBM276092 (5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description The ability of test compounds to inhibit the activity of PHF8 was determined in 384-well plate format under the following reaction conditions: 3 nM P... | US Patent US10174003 (2019) BindingDB Entry DOI: 10.7270/Q2417053 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2B (Homo sapiens (Human)) | BDBM276092 (5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The ability of test compounds to inhibit the activity of FBXL10 was determined in 384-well plate format under the following reaction conditions: 0.3 ... | Bioorg Med Chem Lett 17: 1584-9 (2007) BindingDB Entry DOI: 10.7270/Q2FT8PBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2B (Homo sapiens (Human)) | BDBM276092 (5-[2-(cyclopropylmethoxy)-4-fluorophenyl]-1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description The ability of test compounds to inhibit the activity of FBXL10 was determined in 384-well plate format under the following reaction conditions: 0.3 ... | US Patent US10174003 (2019) BindingDB Entry DOI: 10.7270/Q2417053 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||