

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM278400 (2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-ylpyrrolidin-3-yl]amino]-1,3,4-thiadiazol-2-yl]acetamide::BDBM278426::US10040788, Example 2(a)::US10294221, Example 20(b)
SMILES: CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1
InChI Key: InChIKey=KLQLQDLJJUAEGT-ZBFHGGJFSA-N
Data: 15 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400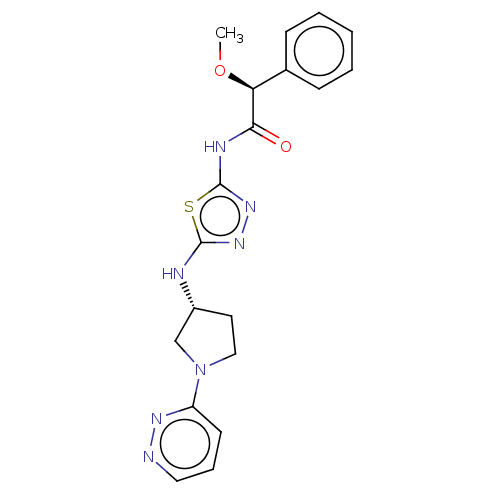 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of OPRM (unknown origin) | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | Bioorg Med Chem Lett 18: 1577-82 (2008) BindingDB Entry DOI: 10.7270/Q2MK6G78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human KGA (63 to 669 residues) preincubated for 15 mins using 50 mM glutamine as substrate by resorufin dye based assay | J Med Chem 62: 46-59 (2019) Article DOI: 10.1021/acs.jmedchem.8b00327 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GLS1 GAC isoform (unknown origin) using glutamine as substrate preincubated for 15 mins followed by substrate addition and measured aft... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by Ionworks electrophysiology assay | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine transporter (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NET (unknown origin) | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of 5HT2B (unknown origin) | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of dopamine transporter (unknown origin) | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NK1R (unknown origin) | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM278400 ((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | 7.8 | n/a |
AstraZeneca AB; Cancer Research Technology Limited US Patent | Assay Description A Glutamate Oxidase/AmplexRed coupled assay was used to measure the ability of compounds to bind to and inhibit the activity of GLS1 in vitro. 6His t... | US Patent US10040788 (2018) BindingDB Entry DOI: 10.7270/Q2Z321PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||