

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM27961 3-(2-benzyl-1H-1,3-benzodiazol-6-yl)-1-[4-(morpholin-4-yl)cyclohexyl]-1H-pyrazolo[3,4-d]pyrimidin-4-amine::3-(2-benzyl-1H-benzo[d]imidazol-5-yl)-1-((1r,4r)-4-morpholinocyclohexyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine::3-(2-benzyl-1H-benzo[d]imidazol-6-yl)-1-((trans)-4-morpholinocyclohexyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine::CHEMBL249496::substituted benzimidazole, 6
SMILES: Nc1ncnc2n(nc(-c3ccc4nc(Cc5ccccc5)[nH]c4c3)c12)[C@H]1CC[C@@H](CC1)N1CCOCC1
InChI Key: InChIKey=MSLPPMUTXCBVLE-HZCBDIJESA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM27961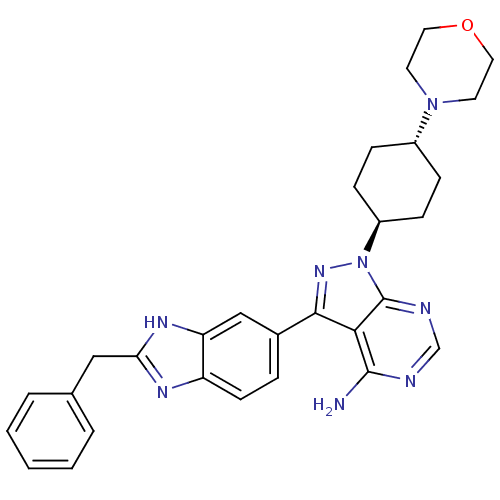 (3-(2-benzyl-1H-1,3-benzodiazol-6-yl)-1-[4-(morphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... | Bioorg Med Chem Lett 19: 1718-21 (2009) Article DOI: 10.1016/j.bmcl.2009.01.086 BindingDB Entry DOI: 10.7270/Q2CJ8BTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EGF-R Tyrosine Kinase Mutant (L858R) (Homo sapiens (Human)) | BDBM27961 (3-(2-benzyl-1H-1,3-benzodiazol-6-yl)-1-[4-(morphol...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... | Bioorg Med Chem Lett 19: 1718-21 (2009) Article DOI: 10.1016/j.bmcl.2009.01.086 BindingDB Entry DOI: 10.7270/Q2CJ8BTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM27961 (3-(2-benzyl-1H-1,3-benzodiazol-6-yl)-1-[4-(morphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human IGF1R expressed in Sf21 cells by time-resolved fluorescence assay | Bioorg Med Chem Lett 17: 5406-9 (2007) Article DOI: 10.1016/j.bmcl.2007.07.037 BindingDB Entry DOI: 10.7270/Q20P0ZRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM27961 (3-(2-benzyl-1H-1,3-benzodiazol-6-yl)-1-[4-(morphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition IGF1R phosphorylation in human MiaPaCa2 cells | Bioorg Med Chem Lett 17: 5406-9 (2007) Article DOI: 10.1016/j.bmcl.2007.07.037 BindingDB Entry DOI: 10.7270/Q20P0ZRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM27961 (3-(2-benzyl-1H-1,3-benzodiazol-6-yl)-1-[4-(morphol...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Abbott Laboratories | Assay Description Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP, and purified kinase in the presence of ... | Bioorg Med Chem Lett 19: 1718-21 (2009) Article DOI: 10.1016/j.bmcl.2009.01.086 BindingDB Entry DOI: 10.7270/Q2CJ8BTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||