

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM283238 4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5,6- dihydropyridyl))-1-methyl-6- oxohydropyrimidin-4-yl]-2- fluorobenzenecarbonitrile::US10023543, Example 26::US10207999, Example 26::US10328077, Example 26::US10548896, Example 26::US10849898, Example 26::US10960005, Example 26::US9573930, Example 26::US9771329, Example 26::US9776974, Example 26
SMILES: COc1ncc(cc1F)-c1c(nc(N2CCC(N)CC2)n(C)c1=O)-c1ccc(C#N)c(F)c1
InChI Key: InChIKey=OFRHYLOQVVXFHG-UHFFFAOYSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238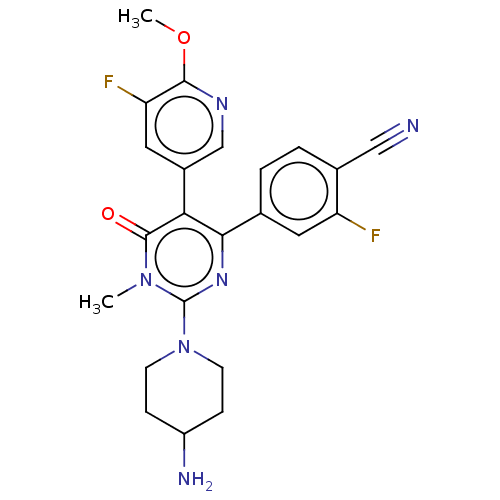 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | 25 |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | US Patent US10023543 (2018) BindingDB Entry DOI: 10.7270/Q2GQ70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | 25 |
Celgene Quanticel Research, Inc. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | US Patent US9573930 (2017) BindingDB Entry DOI: 10.7270/Q2H70HV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description The enzymatic assay of LSD1 activity is based on Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The inhibitory properties ... | US Patent US9776974 (2017) BindingDB Entry DOI: 10.7270/Q2BZ686N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description The enzymatic assay of LSD1 activity is based on Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The inhibitory properties ... | US Patent US9771329 (2017) BindingDB Entry DOI: 10.7270/Q2NG4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | Bioorg Med Chem 17: 7324-36 (2009) BindingDB Entry DOI: 10.7270/Q2W66P3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description The enzymatic assay of LSD-1 activity is based on Tnne Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The inhibitory properties... | US Patent US10960005 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description To analyze LSD1 inhibitor efficacy in cells, a CD11b flow cytometry assay was performed. LSD1 inhibition induces CD11b expression in THP-1 (AML) cell... | US Patent US10207999 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5HRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | US Patent US10543213 (2020) BindingDB Entry DOI: 10.7270/Q2X63QBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | US Patent US10548896 (2020) BindingDB Entry DOI: 10.7270/Q29889CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | US Patent US10849898 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283238 (4-[2-(4-aminopiperidyl)-5-(5- fluoro-6-methoxy(3-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | US Patent US10207999 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5HRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||