

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM284006 E32::US10023564, Example 32
SMILES: CC1=C(C(NC(=O)N1)c1ccc(F)c(CC(=O)N(CCO)Cc2ccco2)c1)C(=O)Nc1ccc2[nH]ncc2c1
InChI Key:
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodopsin kinase (Homo sapiens (Human)) | BDBM284006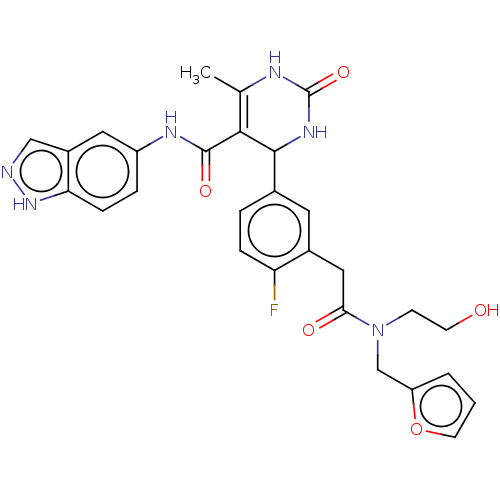 (E32 | US10023564, Example 32) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM284006 (E32 | US10023564, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM284006 (E32 | US10023564, Example 32) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK5 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase (Homo sapiens (Human)) | BDBM284006 (E32 | US10023564, Example 32) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM284006 (E32 | US10023564, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK2 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM284006 (E32 | US10023564, Example 32) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||