

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM28453 (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-[(dimethylamino)methyl]oxolane-3,4-diol::AdoMet substrate analogue, 23b
SMILES: CN(C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12
InChI Key: InChIKey=SLNWRDWGFHZRAQ-WOUKDFQISA-N
PDB links: 1 PDB ID matches this monomer. 10 PDB IDs contain this monomer as substructures. 10 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM28453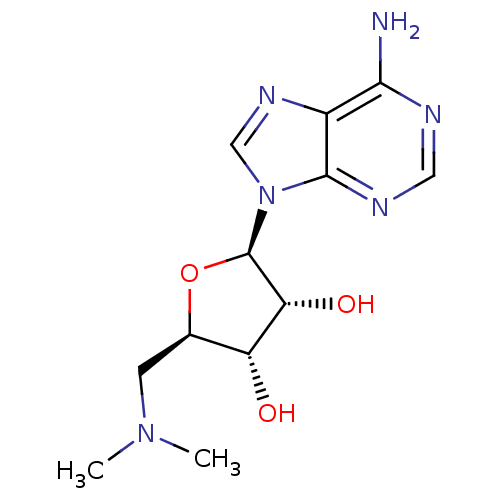 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-[(dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boston University Curated by ChEMBL | Assay Description Inhibition of DOT1-like Histone H3 Methyltransferase (unknown origin) | J Med Chem 62: 10005-10025 (2019) Article DOI: 10.1021/acs.jmedchem.8b01732 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM28453 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-[(dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactor | J Med Chem 56: 8972-83 (2013) Article DOI: 10.1021/jm4007752 BindingDB Entry DOI: 10.7270/Q2D50PDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine decarboxylase 1 (Homo sapiens (Human)) | BDBM28453 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-[(dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||