

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Fc1cc(Cl)ccc1-c1c(nc2cccnn12)C(=O)N1CCC1
InChI Key: InChIKey=FRHMLRQQIOCJKU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 4 of cAMP-specific 3',5'-cyclic phosphodiesterase 4A (RD1) (Homo sapiens (Human)) | BDBM285579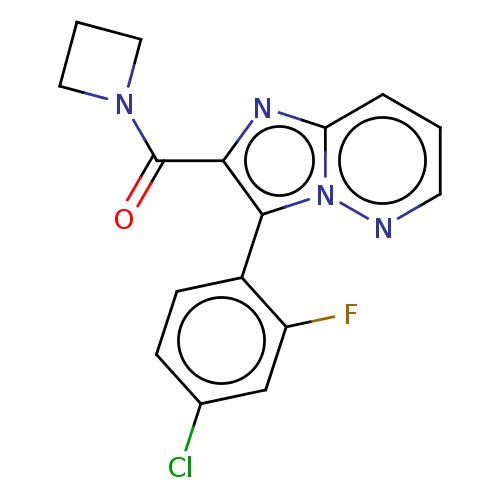 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B [122-736,S654A] (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 3 of cAMP-specific 3',5'-cyclic phosphodiesterase 4D (hPDE4D3) (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 3 of cAMP-specific 3',5'-cyclic phosphodiesterase 4D (hPDE4D3) (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US10077269 (2018) BindingDB Entry DOI: 10.7270/Q2P84DX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... | US Patent US10669279 (2020) BindingDB Entry DOI: 10.7270/Q2765JC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM285579 (US10077269, Example 52 | US10669279, Example 52 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | US Patent US9598421 (2017) BindingDB Entry DOI: 10.7270/Q2K939KG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||