

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC[C@H]1NC(=O)[C@@H]([C@H](O)[C@H](C)Cc2nccn2Cc2ccccn2)N(C)C(=O)[C@@H](C(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](C)NC(=O)[C@@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)N(C)C1=O)C(C)C
InChI Key: InChIKey=YXTRRTPUHABKMO-WZLROUJOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM285697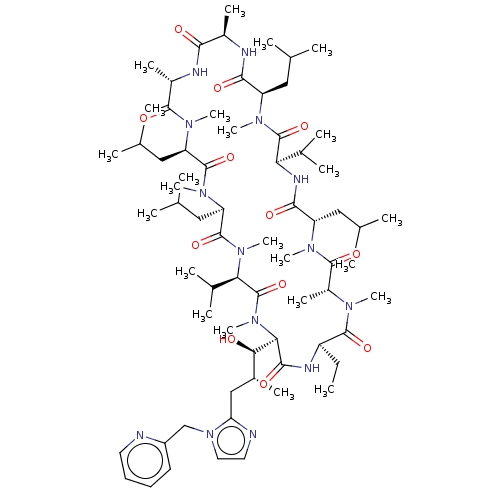 (US10077289, Compound 5 | [(2S,3R,4R)-3-Hydroxy-4-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
Allergan, Inc. US Patent | Assay Description The protease-free PPIase assay measures the rate of cis to trans conversion of a peptide substrate catalyzed by the enzyme cyclophilin A. Addition of... | US Patent US10077289 (2018) BindingDB Entry DOI: 10.7270/Q25B04JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2B catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM285697 (US10077289, Compound 5 | [(2S,3R,4R)-3-Hydroxy-4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan, Inc. US Patent | Assay Description Enzo Life Sciences CaN Assay Kit: BML-AK8042x assay buffer: 100 mM Tris, pH7.5, 200 mM NaCl, 12 mM MgCl2, 1 mM DTT, 0.05% NP-40, 1 mM CaCl2 | US Patent US10077289 (2018) BindingDB Entry DOI: 10.7270/Q25B04JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||